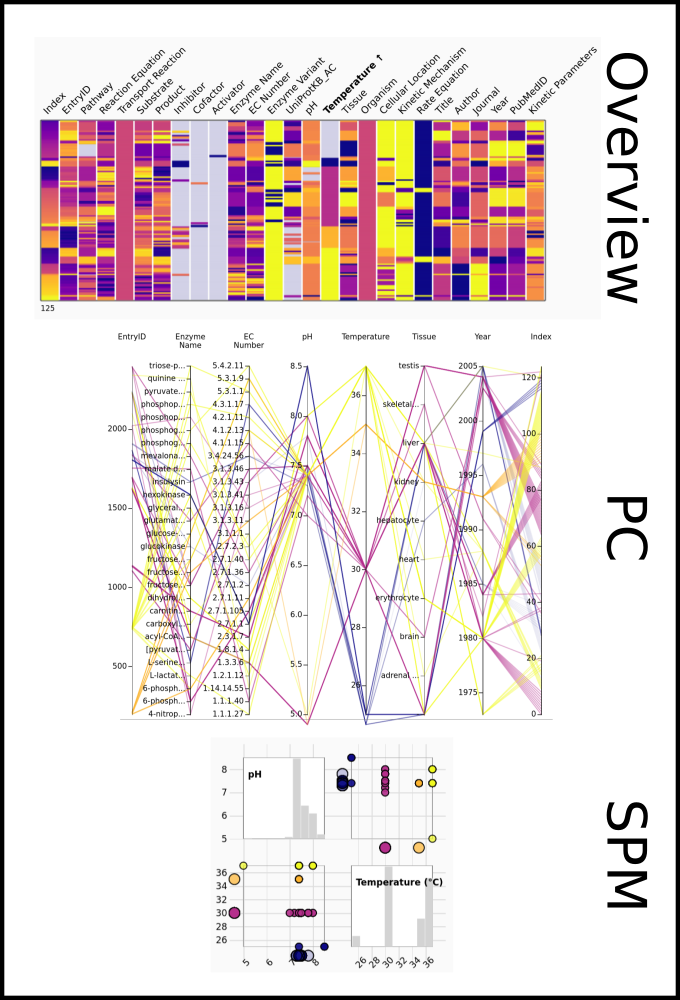
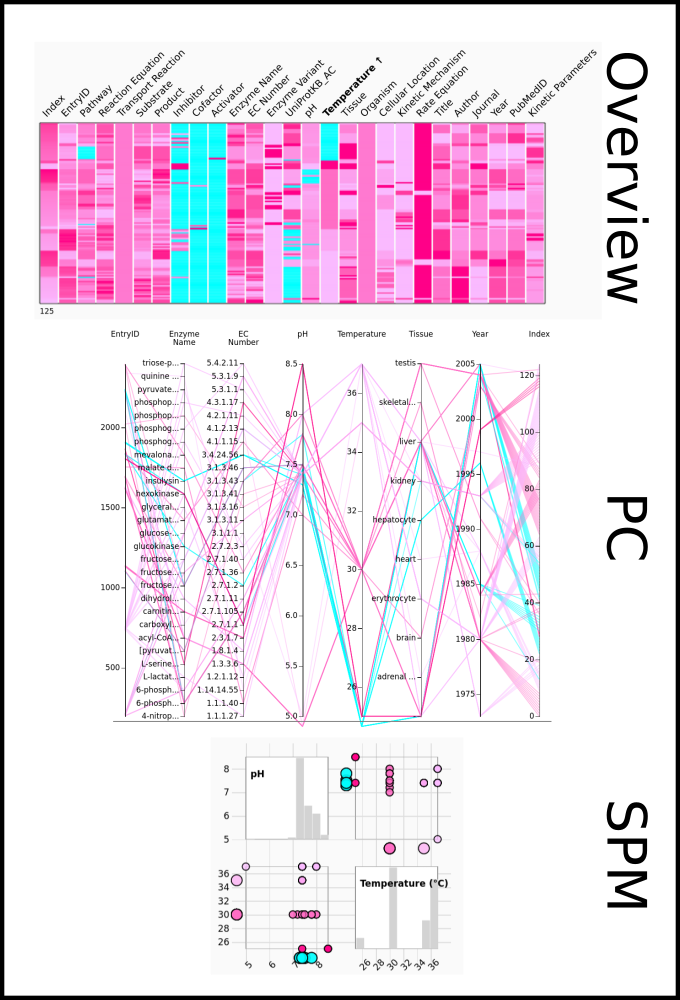
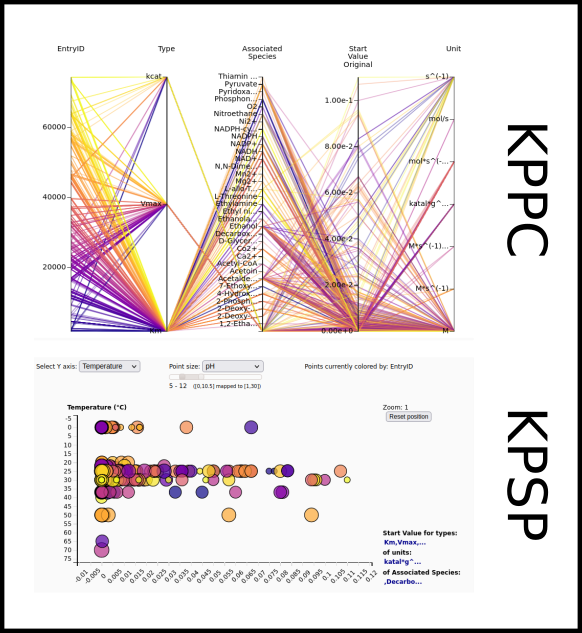
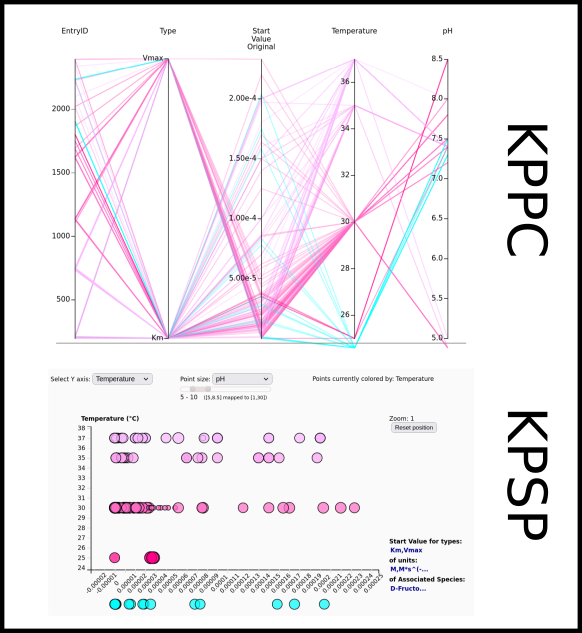
To improve SABIO-RK data interoperability, semantic markup was added to web pages as described and defined by
This structured information makes it easier to discover, collate and analyse our data.
|
6001
|
Identification of the enzymatic active site of CD38 by site-directed mutagenesis
|
|
6002
|
Identification of the enzymatic active site of CD38 by site-directed mutagenesis
|
|
6003
|
Identification of the enzymatic active site of CD38 by site-directed mutagenesis
|
|
6004
|
Identification of the enzymatic active site of CD38 by site-directed mutagenesis
|
|
6005
|
Identification of the enzymatic active site of CD38 by site-directed mutagenesis
|
|
6006
|
Identification of the enzymatic active site of CD38 by site-directed mutagenesis
|
|
6007
|
The G473D mutation impairs dimerization and catalysis in human sulfite oxidase
|
|
6008
|
The G473D mutation impairs dimerization and catalysis in human sulfite oxidase
|
|
6009
|
The G473D mutation impairs dimerization and catalysis in human sulfite oxidase
|
|
6010
|
The G473D mutation impairs dimerization and catalysis in human sulfite oxidase
|
|
6011
|
The G473D mutation impairs dimerization and catalysis in human sulfite oxidase
|
|
6012
|
The G473D mutation impairs dimerization and catalysis in human sulfite oxidase
|
|
6013
|
The G473D mutation impairs dimerization and catalysis in human sulfite oxidase
|
|
6014
|
The G473D mutation impairs dimerization and catalysis in human sulfite oxidase
|
|
6015
|
The G473D mutation impairs dimerization and catalysis in human sulfite oxidase
|
|
6016
|
The G473D mutation impairs dimerization and catalysis in human sulfite oxidase
|
|
6017
|
The G473D mutation impairs dimerization and catalysis in human sulfite oxidase
|
|
6018
|
The G473D mutation impairs dimerization and catalysis in human sulfite oxidase
|
|
6019
|
The G473D mutation impairs dimerization and catalysis in human sulfite oxidase
|
|
6020
|
The G473D mutation impairs dimerization and catalysis in human sulfite oxidase
|
|
6021
|
The G473D mutation impairs dimerization and catalysis in human sulfite oxidase
|
|
6022
|
The G473D mutation impairs dimerization and catalysis in human sulfite oxidase
|
|
6023
|
The G473D mutation impairs dimerization and catalysis in human sulfite oxidase
|
|
6024
|
The G473D mutation impairs dimerization and catalysis in human sulfite oxidase
|
|
6025
|
The G473D mutation impairs dimerization and catalysis in human sulfite oxidase
|
|
6026
|
The G473D mutation impairs dimerization and catalysis in human sulfite oxidase
|
|
6027
|
The G473D mutation impairs dimerization and catalysis in human sulfite oxidase
|
|
6028
|
The G473D mutation impairs dimerization and catalysis in human sulfite oxidase
|
|
6029
|
The G473D mutation impairs dimerization and catalysis in human sulfite oxidase
|
|
6030
|
The G473D mutation impairs dimerization and catalysis in human sulfite oxidase
|
|
6031
|
The G473D mutation impairs dimerization and catalysis in human sulfite oxidase
|
|
6032
|
The G473D mutation impairs dimerization and catalysis in human sulfite oxidase
|
|
6033
|
The G473D mutation impairs dimerization and catalysis in human sulfite oxidase
|
|
6034
|
The G473D mutation impairs dimerization and catalysis in human sulfite oxidase
|
|
6035
|
The G473D mutation impairs dimerization and catalysis in human sulfite oxidase
|
|
6036
|
The G473D mutation impairs dimerization and catalysis in human sulfite oxidase
|
|
6037
|
The G473D mutation impairs dimerization and catalysis in human sulfite oxidase
|
|
6038
|
The G473D mutation impairs dimerization and catalysis in human sulfite oxidase
|
|
6039
|
The G473D mutation impairs dimerization and catalysis in human sulfite oxidase
|
|
6040
|
The G473D mutation impairs dimerization and catalysis in human sulfite oxidase
|
|
6041
|
The G473D mutation impairs dimerization and catalysis in human sulfite oxidase
|
|
6042
|
The G473D mutation impairs dimerization and catalysis in human sulfite oxidase
|
|
6043
|
The G473D mutation impairs dimerization and catalysis in human sulfite oxidase
|
|
6044
|
The G473D mutation impairs dimerization and catalysis in human sulfite oxidase
|
|
6045
|
The G473D mutation impairs dimerization and catalysis in human sulfite oxidase
|
|
6046
|
When a spectator turns killer: suicidal electron transfer from cobalamin in methylmalonyl-CoA mutase
|
|
6047
|
When a spectator turns killer: suicidal electron transfer from cobalamin in methylmalonyl-CoA mutase
|
|
6048
|
Inhibition of human ornithine decarboxylase activity by enantiomers of difluoromethylornithine
|
|
6049
|
Inhibition of human ornithine decarboxylase activity by enantiomers of difluoromethylornithine
|
|
6050
|
Inhibition of human ornithine decarboxylase activity by enantiomers of difluoromethylornithine
|
|
6051
|
Mechanism of Adenylate Kinase. Histidine-36 Is Not Directly Involved in Catalysis, but Protects Cysteine-25 ...
|
|
6052
|
Mechanism of Adenylate Kinase. Histidine-36 Is Not Directly Involved in Catalysis, but Protects Cysteine-25 ...
|
|
6053
|
Mechanism of Adenylate Kinase. Histidine-36 Is Not Directly Involved in Catalysis, but Protects Cysteine-25 ...
|
|
6054
|
Mechanism of Adenylate Kinase. Histidine-36 Is Not Directly Involved in Catalysis, but Protects Cysteine-25 ...
|
|
6055
|
Mechanism of Adenylate Kinase. The Conserved Aspartates 140 and 141 Are Important for Transition State ...
|
|
6056
|
Mechanism of Adenylate Kinase. The Conserved Aspartates 140 and 141 Are Important for Transition State ...
|
|
6057
|
Mechanism of Adenylate Kinase. The Conserved Aspartates 140 and 141 Are Important for Transition State ...
|
|
6058
|
Mechanism of Adenylate Kinase. The Conserved Aspartates 140 and 141 Are Important for Transition State ...
|
|
6059
|
Mechanism of Adenylate Kinase. The Conserved Aspartates 140 and 141 Are Important for Transition State ...
|
|
6060
|
Mechanism of Adenylate Kinase. The Conserved Aspartates 140 and 141 Are Important for Transition State ...
|
|
6061
|
Mechanism of Adenylate Kinase. The Conserved Aspartates 140 and 141 Are Important for Transition State ...
|
|
6062
|
Mechanism of Adenylate Kinase. The Conserved Aspartates 140 and 141 Are Important for Transition State ...
|
|
6063
|
Mechanism of Adenylate Kinase. The Conserved Aspartates 140 and 141 Are Important for Transition State ...
|
|
6064
|
Mechanism of Adenylate Kinase. The Conserved Aspartates 140 and 141 Are Important for Transition State ...
|
|
6065
|
Mechanism of Adenylate Kinase. The Conserved Aspartates 140 and 141 Are Important for Transition State ...
|
|
6066
|
Mechanism of Adenylate Kinase. The Conserved Aspartates 140 and 141 Are Important for Transition State ...
|
|
6067
|
Mechanism of Adenylate Kinase. The Conserved Aspartates 140 and 141 Are Important for Transition State ...
|
|
6068
|
Human aldehyde dehydrogenase 3A1 (ALDH3A1): biochemical characterization and immunohistochemical localization ...
|
|
6069
|
Human aldehyde dehydrogenase 3A1 (ALDH3A1): biochemical characterization and immunohistochemical localization ...
|
|
6070
|
Human aldehyde dehydrogenase 3A1 (ALDH3A1): biochemical characterization and immunohistochemical localization ...
|
|
6071
|
Human aldehyde dehydrogenase 3A1 (ALDH3A1): biochemical characterization and immunohistochemical localization ...
|
|
6072
|
Human aldehyde dehydrogenase 3A1 (ALDH3A1): biochemical characterization and immunohistochemical localization ...
|
|
6073
|
Human aldehyde dehydrogenase 3A1 (ALDH3A1): biochemical characterization and immunohistochemical localization ...
|
|
6074
|
Human aldehyde dehydrogenase 3A1 (ALDH3A1): biochemical characterization and immunohistochemical localization ...
|
|
6075
|
Human aldehyde dehydrogenase 3A1 (ALDH3A1): biochemical characterization and immunohistochemical localization ...
|
|
6076
|
Human aldehyde dehydrogenase 3A1 (ALDH3A1): biochemical characterization and immunohistochemical localization ...
|
|
6077
|
Human aldehyde dehydrogenase 3A1 (ALDH3A1): biochemical characterization and immunohistochemical localization ...
|
|
6078
|
Human aldehyde dehydrogenase 3A1 (ALDH3A1): biochemical characterization and immunohistochemical localization ...
|
|
6079
|
Human aldehyde dehydrogenase 3A1 (ALDH3A1): biochemical characterization and immunohistochemical localization ...
|
|
6080
|
Human aldehyde dehydrogenase 3A1 (ALDH3A1): biochemical characterization and immunohistochemical localization ...
|
|
6081
|
Human aldehyde dehydrogenase 3A1 (ALDH3A1): biochemical characterization and immunohistochemical localization ...
|
|
6082
|
Sulfite:Cytochrome c oxidoreductase from Thiobacillus novellus. Purification, characterization, and molecular ...
|
|
6083
|
Sulfite:Cytochrome c oxidoreductase from Thiobacillus novellus. Purification, characterization, and molecular ...
|
|
6084
|
Sulfite:Cytochrome c oxidoreductase from Thiobacillus novellus. Purification, characterization, and molecular ...
|
|
6085
|
Sulfite:Cytochrome c oxidoreductase from Thiobacillus novellus. Purification, characterization, and molecular ...
|
|
6086
|
Phosphorylation of CTP synthetase on Ser36, Ser330, Ser354, and Ser454 regulates the levels of CTP and ...
|
|
6087
|
Phosphorylation of CTP synthetase on Ser36, Ser330, Ser354, and Ser454 regulates the levels of CTP and ...
|
|
6088
|
Phosphorylation of CTP synthetase on Ser36, Ser330, Ser354, and Ser454 regulates the levels of CTP and ...
|
|
6089
|
Phosphorylation of CTP synthetase on Ser36, Ser330, Ser354, and Ser454 regulates the levels of CTP and ...
|
|
6090
|
Phosphorylation of CTP synthetase on Ser36, Ser330, Ser354, and Ser454 regulates the levels of CTP and ...
|
|
6091
|
Phosphorylation of CTP synthetase on Ser36, Ser330, Ser354, and Ser454 regulates the levels of CTP and ...
|
|
6092
|
Phosphorylation of CTP synthetase on Ser36, Ser330, Ser354, and Ser454 regulates the levels of CTP and ...
|
|
6093
|
Phosphorylation of CTP synthetase on Ser36, Ser330, Ser354, and Ser454 regulates the levels of CTP and ...
|
|
6094
|
Phosphorylation of CTP synthetase on Ser36, Ser330, Ser354, and Ser454 regulates the levels of CTP and ...
|
|
6095
|
Phosphorylation of CTP synthetase on Ser36, Ser330, Ser354, and Ser454 regulates the levels of CTP and ...
|
|
6096
|
Structural and biochemical properties of glycosylated and deglycosylated glucose oxidase from Penicillium ...
|
|
6097
|
Structural and biochemical properties of glycosylated and deglycosylated glucose oxidase from Penicillium ...
|
|
6098
|
Structural and biochemical properties of glycosylated and deglycosylated glucose oxidase from Penicillium ...
|
|
6099
|
Structural and biochemical properties of glycosylated and deglycosylated glucose oxidase from Penicillium ...
|
|
6100
|
Structural and biochemical properties of glycosylated and deglycosylated glucose oxidase from Penicillium ...
|
|
6101
|
Structural and biochemical properties of glycosylated and deglycosylated glucose oxidase from Penicillium ...
|
|
6102
|
Structural and biochemical properties of glycosylated and deglycosylated glucose oxidase from Penicillium ...
|
|
6103
|
Structural and biochemical properties of glycosylated and deglycosylated glucose oxidase from Penicillium ...
|
|
6104
|
Structural and biochemical properties of glycosylated and deglycosylated glucose oxidase from Penicillium ...
|
|
6105
|
Structural and biochemical properties of glycosylated and deglycosylated glucose oxidase from Penicillium ...
|
|
6106
|
Structural and biochemical properties of glycosylated and deglycosylated glucose oxidase from Penicillium ...
|
|
6107
|
Biosynthesis of riboflavin. Studies on the reaction mechanism of 6,7-dimethyl-8-ribityllumazine synthase
|
|
6108
|
Biosynthesis of riboflavin. Studies on the reaction mechanism of 6,7-dimethyl-8-ribityllumazine synthase
|
|
6109
|
Biosynthesis of riboflavin. Studies on the reaction mechanism of 6,7-dimethyl-8-ribityllumazine synthase
|
|
6110
|
Biosynthesis of riboflavin. Studies on the reaction mechanism of 6,7-dimethyl-8-ribityllumazine synthase
|
|
6111
|
Biosynthesis of riboflavin. Studies on the reaction mechanism of 6,7-dimethyl-8-ribityllumazine synthase
|
|
6112
|
Subcellular compartmentation and differential catalytic properties of the three human nicotinamide ...
|
|
6113
|
Subcellular compartmentation and differential catalytic properties of the three human nicotinamide ...
|
|
6114
|
Subcellular compartmentation and differential catalytic properties of the three human nicotinamide ...
|
|
6115
|
Subcellular compartmentation and differential catalytic properties of the three human nicotinamide ...
|
|
6116
|
Subcellular compartmentation and differential catalytic properties of the three human nicotinamide ...
|
|
6117
|
Subcellular compartmentation and differential catalytic properties of the three human nicotinamide ...
|
|
6118
|
Subcellular compartmentation and differential catalytic properties of the three human nicotinamide ...
|
|
6119
|
Subcellular compartmentation and differential catalytic properties of the three human nicotinamide ...
|
|
6120
|
Subcellular compartmentation and differential catalytic properties of the three human nicotinamide ...
|
|
6121
|
Subcellular compartmentation and differential catalytic properties of the three human nicotinamide ...
|
|
6122
|
Subcellular compartmentation and differential catalytic properties of the three human nicotinamide ...
|
|
6123
|
Subcellular compartmentation and differential catalytic properties of the three human nicotinamide ...
|
|
6124
|
Dimeric 3-phosphoglycerate kinases from hyperthermophilic Archaea. Cloning, sequencing and expression of the ...
|
|
6125
|
Dimeric 3-phosphoglycerate kinases from hyperthermophilic Archaea. Cloning, sequencing and expression of the ...
|
|
6126
|
Dimeric 3-phosphoglycerate kinases from hyperthermophilic Archaea. Cloning, sequencing and expression of the ...
|
|
6127
|
Dimeric 3-phosphoglycerate kinases from hyperthermophilic Archaea. Cloning, sequencing and expression of the ...
|
|
6128
|
Dimeric 3-phosphoglycerate kinases from hyperthermophilic Archaea. Cloning, sequencing and expression of the ...
|
|
6129
|
Dimeric 3-phosphoglycerate kinases from hyperthermophilic Archaea. Cloning, sequencing and expression of the ...
|
|
6130
|
Dimeric 3-phosphoglycerate kinases from hyperthermophilic Archaea. Cloning, sequencing and expression of the ...
|
|
6131
|
Dimeric 3-phosphoglycerate kinases from hyperthermophilic Archaea. Cloning, sequencing and expression of the ...
|
|
6132
|
Dimeric 3-phosphoglycerate kinases from hyperthermophilic Archaea. Cloning, sequencing and expression of the ...
|
|
6133
|
Dimeric 3-phosphoglycerate kinases from hyperthermophilic Archaea. Cloning, sequencing and expression of the ...
|
|
6134
|
Dimeric 3-phosphoglycerate kinases from hyperthermophilic Archaea. Cloning, sequencing and expression of the ...
|
|
6135
|
A catalytic triad is responsible for acid-base chemistry in the ascaris suum NAD-malic enzyme
|
|
6136
|
A catalytic triad is responsible for acid-base chemistry in the ascaris suum NAD-malic enzyme
|
|
6137
|
A catalytic triad is responsible for acid-base chemistry in the ascaris suum NAD-malic enzyme
|
|
6138
|
Kinetic regulation of the mitochondrial glycerol-3-phosphate dehydrogenase by the external NADH dehydrogenase ...
|
|
6139
|
Kinetic regulation of the mitochondrial glycerol-3-phosphate dehydrogenase by the external NADH dehydrogenase ...
|
|
6140
|
Kinetic regulation of the mitochondrial glycerol-3-phosphate dehydrogenase by the external NADH dehydrogenase ...
|
|
6141
|
Kinetic regulation of the mitochondrial glycerol-3-phosphate dehydrogenase by the external NADH dehydrogenase ...
|
|
6142
|
Acid-base catalysis in the extradiol catechol dioxygenase reaction mechanism: site-directed mutagenesis of ...
|
|
6143
|
Acid-base catalysis in the extradiol catechol dioxygenase reaction mechanism: site-directed mutagenesis of ...
|
|
6144
|
Acid-base catalysis in the extradiol catechol dioxygenase reaction mechanism: site-directed mutagenesis of ...
|
|
6145
|
Acid-base catalysis in the extradiol catechol dioxygenase reaction mechanism: site-directed mutagenesis of ...
|
|
6146
|
Acid-base catalysis in the extradiol catechol dioxygenase reaction mechanism: site-directed mutagenesis of ...
|
|
6147
|
A bifunctional dCTP deaminase-dUTP nucleotidohydrolase from the hyperthermophilic archaeon Methanocaldococcus ...
|
|
6148
|
A bifunctional dCTP deaminase-dUTP nucleotidohydrolase from the hyperthermophilic archaeon Methanocaldococcus ...
|
|
6149
|
A bifunctional dCTP deaminase-dUTP nucleotidohydrolase from the hyperthermophilic archaeon Methanocaldococcus ...
|
|
6150
|
A bifunctional dCTP deaminase-dUTP nucleotidohydrolase from the hyperthermophilic archaeon Methanocaldococcus ...
|
|
6151
|
A bifunctional dCTP deaminase-dUTP nucleotidohydrolase from the hyperthermophilic archaeon Methanocaldococcus ...
|
|
6152
|
A bifunctional dCTP deaminase-dUTP nucleotidohydrolase from the hyperthermophilic archaeon Methanocaldococcus ...
|
|
6153
|
A bifunctional dCTP deaminase-dUTP nucleotidohydrolase from the hyperthermophilic archaeon Methanocaldococcus ...
|
|
6154
|
A bifunctional dCTP deaminase-dUTP nucleotidohydrolase from the hyperthermophilic archaeon Methanocaldococcus ...
|
|
6155
|
The Active Site Topology of Aspergillus niger Endopolygalacturonase II as Studied by Site-directed Mutagenesis
|
|
6156
|
The Active Site Topology of Aspergillus niger Endopolygalacturonase II as Studied by Site-directed Mutagenesis
|
|
6157
|
The Active Site Topology of Aspergillus niger Endopolygalacturonase II as Studied by Site-directed Mutagenesis
|
|
6158
|
The Active Site Topology of Aspergillus niger Endopolygalacturonase II as Studied by Site-directed Mutagenesis
|
|
6159
|
The Active Site Topology of Aspergillus niger Endopolygalacturonase II as Studied by Site-directed Mutagenesis
|
|
6160
|
The Active Site Topology of Aspergillus niger Endopolygalacturonase II as Studied by Site-directed Mutagenesis
|
|
6161
|
The Active Site Topology of Aspergillus niger Endopolygalacturonase II as Studied by Site-directed Mutagenesis
|
|
6162
|
The Active Site Topology of Aspergillus niger Endopolygalacturonase II as Studied by Site-directed Mutagenesis
|
|
6163
|
The Active Site Topology of Aspergillus niger Endopolygalacturonase II as Studied by Site-directed Mutagenesis
|
|
6164
|
The Active Site Topology of Aspergillus niger Endopolygalacturonase II as Studied by Site-directed Mutagenesis
|
|
6165
|
The Active Site Topology of Aspergillus niger Endopolygalacturonase II as Studied by Site-directed Mutagenesis
|
|
6166
|
The Active Site Topology of Aspergillus niger Endopolygalacturonase II as Studied by Site-directed Mutagenesis
|
|
6167
|
The Active Site Topology of Aspergillus niger Endopolygalacturonase II as Studied by Site-directed Mutagenesis
|
|
6168
|
The Active Site Topology of Aspergillus niger Endopolygalacturonase II as Studied by Site-directed Mutagenesis
|
|
6169
|
The Active Site Topology of Aspergillus niger Endopolygalacturonase II as Studied by Site-directed Mutagenesis
|
|
6170
|
Subsite mapping of Aspergillus niger Endopolygalacturonase II by site-directed mutagenesis
|
|
6171
|
Subsite mapping of Aspergillus niger Endopolygalacturonase II by site-directed mutagenesis
|
|
6172
|
Subsite mapping of Aspergillus niger Endopolygalacturonase II by site-directed mutagenesis
|
|
6173
|
Subsite mapping of Aspergillus niger Endopolygalacturonase II by site-directed mutagenesis
|
|
6174
|
Subsite mapping of Aspergillus niger Endopolygalacturonase II by site-directed mutagenesis
|
|
6175
|
Subsite mapping of Aspergillus niger Endopolygalacturonase II by site-directed mutagenesis
|
|
6176
|
Subsite mapping of Aspergillus niger Endopolygalacturonase II by site-directed mutagenesis
|
|
6177
|
Subsite mapping of Aspergillus niger Endopolygalacturonase II by site-directed mutagenesis
|
|
6178
|
Subsite mapping of Aspergillus niger Endopolygalacturonase II by site-directed mutagenesis
|
|
6179
|
Subsite mapping of Aspergillus niger Endopolygalacturonase II by site-directed mutagenesis
|
|
6180
|
Phosphoglycerate mutase from wheat germ: studies with isotopically labeled 3-phospho-D-glycerates showing that ...
|
|
6181
|
Molecular cloning, expression, and characterization of adenylate isopentenyltransferase from hop (Humulus ...
|
|
6182
|
Molecular cloning, expression, and characterization of adenylate isopentenyltransferase from hop (Humulus ...
|
|
6183
|
Molecular cloning, expression, and characterization of adenylate isopentenyltransferase from hop (Humulus ...
|
|
6184
|
Molecular cloning, expression, and characterization of adenylate isopentenyltransferase from hop (Humulus ...
|
|
6185
|
A comparison of the reactivity and stability of wild type and His388----Gln mutant phosphoglycerate kinase ...
|
|
6186
|
A comparison of the reactivity and stability of wild type and His388----Gln mutant phosphoglycerate kinase ...
|
|
6187
|
Site-directed mutagenesis of histidine-388 in the hinge region of yeast 3-phosphoglycerate kinase: effects on ...
|
|
6188
|
Site-directed mutagenesis of histidine-388 in the hinge region of yeast 3-phosphoglycerate kinase: effects on ...
|
|
6189
|
Site-directed mutagenesis of histidine-388 in the hinge region of yeast 3-phosphoglycerate kinase: effects on ...
|
|
6190
|
Site-directed mutagenesis of histidine-388 in the hinge region of yeast 3-phosphoglycerate kinase: effects on ...
|
|
6191
|
Site-directed mutagenesis of histidine-388 in the hinge region of yeast 3-phosphoglycerate kinase: effects on ...
|
|
6192
|
Site-directed mutagenesis of histidine-388 in the hinge region of yeast 3-phosphoglycerate kinase: effects on ...
|
|
6193
|
Duck liver 'malic' enzyme. Expression in Escherichia coli and characterization of the wild-type enzyme and ...
|
|
6194
|
Duck liver 'malic' enzyme. Expression in Escherichia coli and characterization of the wild-type enzyme and ...
|
|
6195
|
Duck liver 'malic' enzyme. Expression in Escherichia coli and characterization of the wild-type enzyme and ...
|
|
6196
|
Duck liver 'malic' enzyme. Expression in Escherichia coli and characterization of the wild-type enzyme and ...
|
|
6197
|
An NMR analysis of the binding of inhibitors to yeast phosphoglycerate kinase
|
|
6198
|
An NMR analysis of the binding of inhibitors to yeast phosphoglycerate kinase
|
|
6199
|
An NMR analysis of the binding of inhibitors to yeast phosphoglycerate kinase
|
|
6200
|
An NMR analysis of the binding of inhibitors to yeast phosphoglycerate kinase
|
|
6201
|
An NMR analysis of the binding of inhibitors to yeast phosphoglycerate kinase
|
|
6202
|
An NMR analysis of the binding of inhibitors to yeast phosphoglycerate kinase
|
|
6203
|
An NMR analysis of the binding of inhibitors to yeast phosphoglycerate kinase
|
|
6204
|
The relationship between side reactions and slow inhibition of ribulose-bisphosphate carboxylase revealed by a ...
|
|
6205
|
The relationship between side reactions and slow inhibition of ribulose-bisphosphate carboxylase revealed by a ...
|
|
6206
|
The relationship between side reactions and slow inhibition of ribulose-bisphosphate carboxylase revealed by a ...
|
|
6207
|
The relationship between side reactions and slow inhibition of ribulose-bisphosphate carboxylase revealed by a ...
|
|
6208
|
Molecular evolution by change of function. Alkaloid-specific homospermidine synthase retained all properties ...
|
|
6209
|
Molecular evolution by change of function. Alkaloid-specific homospermidine synthase retained all properties ...
|
|
6210
|
Molecular evolution by change of function. Alkaloid-specific homospermidine synthase retained all properties ...
|
|
6211
|
Molecular evolution by change of function. Alkaloid-specific homospermidine synthase retained all properties ...
|
|
6212
|
Molecular evolution by change of function. Alkaloid-specific homospermidine synthase retained all properties ...
|
|
6213
|
Molecular evolution by change of function. Alkaloid-specific homospermidine synthase retained all properties ...
|
|
6214
|
Molecular evolution by change of function. Alkaloid-specific homospermidine synthase retained all properties ...
|
|
6215
|
Molecular evolution by change of function. Alkaloid-specific homospermidine synthase retained all properties ...
|
|
6216
|
Molecular evolution by change of function. Alkaloid-specific homospermidine synthase retained all properties ...
|
|
6217
|
Molecular evolution by change of function. Alkaloid-specific homospermidine synthase retained all properties ...
|
|
6218
|
Molecular evolution by change of function. Alkaloid-specific homospermidine synthase retained all properties ...
|
|
6219
|
Molecular evolution by change of function. Alkaloid-specific homospermidine synthase retained all properties ...
|
|
6220
|
Molecular evolution by change of function. Alkaloid-specific homospermidine synthase retained all properties ...
|
|
6221
|
Molecular evolution by change of function. Alkaloid-specific homospermidine synthase retained all properties ...
|
|
6222
|
Molecular evolution by change of function. Alkaloid-specific homospermidine synthase retained all properties ...
|
|
6223
|
Molecular evolution by change of function. Alkaloid-specific homospermidine synthase retained all properties ...
|
|
6224
|
Molecular evolution by change of function. Alkaloid-specific homospermidine synthase retained all properties ...
|
|
6225
|
Molecular evolution by change of function. Alkaloid-specific homospermidine synthase retained all properties ...
|
|
6226
|
Molecular evolution by change of function. Alkaloid-specific homospermidine synthase retained all properties ...
|
|
6227
|
Aldh3a1 protects human corneal epithelial cells from ultraviolet- and 4-hydroxy-2-nonenal-induced oxidative ...
|
|
6228
|
Aldh3a1 protects human corneal epithelial cells from ultraviolet- and 4-hydroxy-2-nonenal-induced oxidative ...
|
|
6229
|
Aldh3a1 protects human corneal epithelial cells from ultraviolet- and 4-hydroxy-2-nonenal-induced oxidative ...
|
|
6230
|
Aldh3a1 protects human corneal epithelial cells from ultraviolet- and 4-hydroxy-2-nonenal-induced oxidative ...
|
|
6231
|
Aldh3a1 protects human corneal epithelial cells from ultraviolet- and 4-hydroxy-2-nonenal-induced oxidative ...
|
|
6232
|
Aldh3a1 protects human corneal epithelial cells from ultraviolet- and 4-hydroxy-2-nonenal-induced oxidative ...
|
|
6233
|
Aldh3a1 protects human corneal epithelial cells from ultraviolet- and 4-hydroxy-2-nonenal-induced oxidative ...
|
|
6234
|
Aldh3a1 protects human corneal epithelial cells from ultraviolet- and 4-hydroxy-2-nonenal-induced oxidative ...
|
|
6235
|
Aldh3a1 protects human corneal epithelial cells from ultraviolet- and 4-hydroxy-2-nonenal-induced oxidative ...
|
|
6236
|
Altering substrate specificity of Bacillus sp. SAM1606 alpha-Glucosidase by comparative site-specific ...
|
|
6237
|
Altering substrate specificity of Bacillus sp. SAM1606 alpha-Glucosidase by comparative site-specific ...
|
|
6238
|
Altering substrate specificity of Bacillus sp. SAM1606 alpha-Glucosidase by comparative site-specific ...
|
|
6239
|
Altering substrate specificity of Bacillus sp. SAM1606 alpha-Glucosidase by comparative site-specific ...
|
|
6240
|
Altering substrate specificity of Bacillus sp. SAM1606 alpha-Glucosidase by comparative site-specific ...
|
|
6241
|
Altering substrate specificity of Bacillus sp. SAM1606 alpha-Glucosidase by comparative site-specific ...
|
|
6242
|
Altering substrate specificity of Bacillus sp. SAM1606 alpha-Glucosidase by comparative site-specific ...
|
|
6243
|
Altering substrate specificity of Bacillus sp. SAM1606 alpha-Glucosidase by comparative site-specific ...
|
|
6244
|
Altering substrate specificity of Bacillus sp. SAM1606 alpha-Glucosidase by comparative site-specific ...
|
|
6245
|
Altering substrate specificity of Bacillus sp. SAM1606 alpha-Glucosidase by comparative site-specific ...
|
|
6246
|
Altering substrate specificity of Bacillus sp. SAM1606 alpha-Glucosidase by comparative site-specific ...
|
|
6247
|
Altering substrate specificity of Bacillus sp. SAM1606 alpha-Glucosidase by comparative site-specific ...
|
|
6248
|
Altering substrate specificity of Bacillus sp. SAM1606 alpha-Glucosidase by comparative site-specific ...
|
|
6249
|
Altering substrate specificity of Bacillus sp. SAM1606 alpha-Glucosidase by comparative site-specific ...
|
|
6250
|
Altering substrate specificity of Bacillus sp. SAM1606 alpha-Glucosidase by comparative site-specific ...
|
|
6251
|
Altering substrate specificity of Bacillus sp. SAM1606 alpha-Glucosidase by comparative site-specific ...
|
|
6252
|
Altering substrate specificity of Bacillus sp. SAM1606 alpha-Glucosidase by comparative site-specific ...
|
|
6253
|
Altering substrate specificity of Bacillus sp. SAM1606 alpha-Glucosidase by comparative site-specific ...
|
|
6254
|
Altering substrate specificity of Bacillus sp. SAM1606 alpha-Glucosidase by comparative site-specific ...
|
|
6255
|
Altering substrate specificity of Bacillus sp. SAM1606 alpha-Glucosidase by comparative site-specific ...
|
|
6256
|
Altering substrate specificity of Bacillus sp. SAM1606 alpha-Glucosidase by comparative site-specific ...
|
|
6257
|
Altering substrate specificity of Bacillus sp. SAM1606 alpha-Glucosidase by comparative site-specific ...
|
|
6258
|
Altering substrate specificity of Bacillus sp. SAM1606 alpha-Glucosidase by comparative site-specific ...
|
|
6259
|
Altering substrate specificity of Bacillus sp. SAM1606 alpha-Glucosidase by comparative site-specific ...
|
|
6260
|
Altering substrate specificity of Bacillus sp. SAM1606 alpha-Glucosidase by comparative site-specific ...
|
|
6261
|
Altering substrate specificity of Bacillus sp. SAM1606 alpha-Glucosidase by comparative site-specific ...
|
|
6262
|
Altering substrate specificity of Bacillus sp. SAM1606 alpha-Glucosidase by comparative site-specific ...
|
|
6263
|
Altering substrate specificity of Bacillus sp. SAM1606 alpha-Glucosidase by comparative site-specific ...
|
|
6264
|
Altering substrate specificity of Bacillus sp. SAM1606 alpha-Glucosidase by comparative site-specific ...
|
|
6265
|
Altering substrate specificity of Bacillus sp. SAM1606 alpha-Glucosidase by comparative site-specific ...
|
|
6266
|
Altering substrate specificity of Bacillus sp. SAM1606 alpha-Glucosidase by comparative site-specific ...
|
|
6267
|
Altering substrate specificity of Bacillus sp. SAM1606 alpha-Glucosidase by comparative site-specific ...
|
|
6268
|
Altering substrate specificity of Bacillus sp. SAM1606 alpha-Glucosidase by comparative site-specific ...
|
|
6269
|
Altering substrate specificity of Bacillus sp. SAM1606 alpha-Glucosidase by comparative site-specific ...
|
|
6270
|
Altering substrate specificity of Bacillus sp. SAM1606 alpha-Glucosidase by comparative site-specific ...
|
|
6271
|
Altering substrate specificity of Bacillus sp. SAM1606 alpha-Glucosidase by comparative site-specific ...
|
|
6272
|
Altering substrate specificity of Bacillus sp. SAM1606 alpha-Glucosidase by comparative site-specific ...
|
|
6273
|
Altering substrate specificity of Bacillus sp. SAM1606 alpha-Glucosidase by comparative site-specific ...
|
|
6274
|
Altering substrate specificity of Bacillus sp. SAM1606 alpha-Glucosidase by comparative site-specific ...
|
|
6275
|
Altering substrate specificity of Bacillus sp. SAM1606 alpha-Glucosidase by comparative site-specific ...
|
|
6276
|
Altering substrate specificity of Bacillus sp. SAM1606 alpha-Glucosidase by comparative site-specific ...
|
|
6277
|
Altering substrate specificity of Bacillus sp. SAM1606 alpha-Glucosidase by comparative site-specific ...
|
|
6278
|
Crystallization and properties of pea glucosephosphate isomerase
|
|
6279
|
Fructose-6-phosphate and AMP; effectors of proline biosynthesis in Escherichia coli
|
|
6280
|
Mitochondrial trans-2-enoyl-CoA reductase of wax ester fermentation from Euglena gracilis defines a new family ...
|
|
6281
|
Mitochondrial trans-2-enoyl-CoA reductase of wax ester fermentation from Euglena gracilis defines a new family ...
|
|
6282
|
Mitochondrial trans-2-enoyl-CoA reductase of wax ester fermentation from Euglena gracilis defines a new family ...
|
|
6283
|
Mitochondrial trans-2-enoyl-CoA reductase of wax ester fermentation from Euglena gracilis defines a new family ...
|
|
6284
|
Mitochondrial trans-2-enoyl-CoA reductase of wax ester fermentation from Euglena gracilis defines a new family ...
|
|
6285
|
Mechanism of nicotinamide inhibition and transglycosidation by Sir2 histone/protein deacetylases
|
|
6286
|
Mechanism of nicotinamide inhibition and transglycosidation by Sir2 histone/protein deacetylases
|
|
6287
|
Mechanism of nicotinamide inhibition and transglycosidation by Sir2 histone/protein deacetylases
|
|
6288
|
Mechanism of nicotinamide inhibition and transglycosidation by Sir2 histone/protein deacetylases
|
|
6289
|
Mechanism of nicotinamide inhibition and transglycosidation by Sir2 histone/protein deacetylases
|
|
6290
|
Mechanism of nicotinamide inhibition and transglycosidation by Sir2 histone/protein deacetylases
|
|
6291
|
Mechanism of nicotinamide inhibition and transglycosidation by Sir2 histone/protein deacetylases
|
|
6292
|
Mechanism of nicotinamide inhibition and transglycosidation by Sir2 histone/protein deacetylases
|
|
6293
|
Mechanism of nicotinamide inhibition and transglycosidation by Sir2 histone/protein deacetylases
|
|
6294
|
Mechanism of nicotinamide inhibition and transglycosidation by Sir2 histone/protein deacetylases
|
|
6295
|
Mechanism of nicotinamide inhibition and transglycosidation by Sir2 histone/protein deacetylases
|
|
6296
|
Purification, cloning, and expression of the mitochondrial malate dehydrogenase (mMDH) from protoscolices of ...
|
|
6297
|
Purification, cloning, and expression of the mitochondrial malate dehydrogenase (mMDH) from protoscolices of ...
|
|
6298
|
Purification, cloning, and expression of the mitochondrial malate dehydrogenase (mMDH) from protoscolices of ...
|
|
6299
|
Purification, cloning, and expression of the mitochondrial malate dehydrogenase (mMDH) from protoscolices of ...
|
|
6300
|
Purification, cloning, and expression of the mitochondrial malate dehydrogenase (mMDH) from protoscolices of ...
|
|
6301
|
Purification, cloning, and expression of the mitochondrial malate dehydrogenase (mMDH) from protoscolices of ...
|
|
6302
|
Purification, cloning, and expression of the mitochondrial malate dehydrogenase (mMDH) from protoscolices of ...
|
|
6303
|
Purification, cloning, and expression of the mitochondrial malate dehydrogenase (mMDH) from protoscolices of ...
|
|
6304
|
Purification, cloning, and expression of the mitochondrial malate dehydrogenase (mMDH) from protoscolices of ...
|
|
6305
|
Purification, cloning, and expression of the mitochondrial malate dehydrogenase (mMDH) from protoscolices of ...
|
|
6306
|
Purification, cloning, and expression of the mitochondrial malate dehydrogenase (mMDH) from protoscolices of ...
|
|
6307
|
Purification, cloning, and expression of the mitochondrial malate dehydrogenase (mMDH) from protoscolices of ...
|
|
6308
|
Kinetic study of the oxidation of quercetin by mushroom tyrosinase
|
|
6309
|
Kinetic study of the oxidation of quercetin by mushroom tyrosinase
|
|
6310
|
Kinetic study of the oxidation of quercetin by mushroom tyrosinase
|
|
6311
|
Purification and characterization of malate dehydrogenase from Corynebacterium glutamicum
|
|
6312
|
Purification and characterization of malate dehydrogenase from Corynebacterium glutamicum
|
|
6313
|
Purification and characterization of malate dehydrogenase from Corynebacterium glutamicum
|
|
6314
|
Purification and characterization of malate dehydrogenase from Corynebacterium glutamicum
|
|
6315
|
Purification and characterization of malate dehydrogenase from Corynebacterium glutamicum
|
|
6316
|
Purification and characterization of malate dehydrogenase from Corynebacterium glutamicum
|
|
6317
|
Purification and characterization of malate dehydrogenase from Corynebacterium glutamicum
|
|
6318
|
Purification and characterization of malate dehydrogenase from Corynebacterium glutamicum
|
|
6319
|
Purification and characterization of malate dehydrogenase from Corynebacterium glutamicum
|
|
6320
|
Purification and characterization of malate dehydrogenase from Corynebacterium glutamicum
|
|
6321
|
Purification and characterization of malate dehydrogenase from Corynebacterium glutamicum
|
|
6322
|
Cupin-type phosphoglucose isomerases (Cupin-PGIs) constitute a novel metal-dependent PGI family representing a ...
|
|
6323
|
Cupin-type phosphoglucose isomerases (Cupin-PGIs) constitute a novel metal-dependent PGI family representing a ...
|
|
6324
|
Cupin-type phosphoglucose isomerases (Cupin-PGIs) constitute a novel metal-dependent PGI family representing a ...
|
|
6325
|
Cupin-type phosphoglucose isomerases (Cupin-PGIs) constitute a novel metal-dependent PGI family representing a ...
|
|
6326
|
Cupin-type phosphoglucose isomerases (Cupin-PGIs) constitute a novel metal-dependent PGI family representing a ...
|
|
6327
|
Cupin-type phosphoglucose isomerases (Cupin-PGIs) constitute a novel metal-dependent PGI family representing a ...
|
|
6328
|
Cupin-type phosphoglucose isomerases (Cupin-PGIs) constitute a novel metal-dependent PGI family representing a ...
|
|
6329
|
Cupin-type phosphoglucose isomerases (Cupin-PGIs) constitute a novel metal-dependent PGI family representing a ...
|
|
6330
|
Cupin-type phosphoglucose isomerases (Cupin-PGIs) constitute a novel metal-dependent PGI family representing a ...
|
|
6331
|
Cupin-type phosphoglucose isomerases (Cupin-PGIs) constitute a novel metal-dependent PGI family representing a ...
|
|
6332
|
Cupin-type phosphoglucose isomerases (Cupin-PGIs) constitute a novel metal-dependent PGI family representing a ...
|
|
6333
|
Cupin-type phosphoglucose isomerases (Cupin-PGIs) constitute a novel metal-dependent PGI family representing a ...
|
|
6334
|
Cupin-type phosphoglucose isomerases (Cupin-PGIs) constitute a novel metal-dependent PGI family representing a ...
|
|
6335
|
Cupin-type phosphoglucose isomerases (Cupin-PGIs) constitute a novel metal-dependent PGI family representing a ...
|
|
6336
|
Cupin-type phosphoglucose isomerases (Cupin-PGIs) constitute a novel metal-dependent PGI family representing a ...
|
|
6337
|
Cupin-type phosphoglucose isomerases (Cupin-PGIs) constitute a novel metal-dependent PGI family representing a ...
|
|
6338
|
Cupin-type phosphoglucose isomerases (Cupin-PGIs) constitute a novel metal-dependent PGI family representing a ...
|
|
6339
|
Cupin-type phosphoglucose isomerases (Cupin-PGIs) constitute a novel metal-dependent PGI family representing a ...
|
|
6340
|
Cupin-type phosphoglucose isomerases (Cupin-PGIs) constitute a novel metal-dependent PGI family representing a ...
|
|
6341
|
Cupin-type phosphoglucose isomerases (Cupin-PGIs) constitute a novel metal-dependent PGI family representing a ...
|
|
6342
|
Cupin-type phosphoglucose isomerases (Cupin-PGIs) constitute a novel metal-dependent PGI family representing a ...
|
|
6343
|
Cupin-type phosphoglucose isomerases (Cupin-PGIs) constitute a novel metal-dependent PGI family representing a ...
|
|
6344
|
Cupin-type phosphoglucose isomerases (Cupin-PGIs) constitute a novel metal-dependent PGI family representing a ...
|
|
6345
|
Cupin-type phosphoglucose isomerases (Cupin-PGIs) constitute a novel metal-dependent PGI family representing a ...
|
|
6346
|
Cupin-type phosphoglucose isomerases (Cupin-PGIs) constitute a novel metal-dependent PGI family representing a ...
|
|
6347
|
Cupin-type phosphoglucose isomerases (Cupin-PGIs) constitute a novel metal-dependent PGI family representing a ...
|
|
6348
|
Cupin-type phosphoglucose isomerases (Cupin-PGIs) constitute a novel metal-dependent PGI family representing a ...
|
|
6349
|
Cupin-type phosphoglucose isomerases (Cupin-PGIs) constitute a novel metal-dependent PGI family representing a ...
|
|
6350
|
Cupin-type phosphoglucose isomerases (Cupin-PGIs) constitute a novel metal-dependent PGI family representing a ...
|
|
6351
|
Cupin-type phosphoglucose isomerases (Cupin-PGIs) constitute a novel metal-dependent PGI family representing a ...
|
|
6352
|
Cupin-type phosphoglucose isomerases (Cupin-PGIs) constitute a novel metal-dependent PGI family representing a ...
|
|
6353
|
Cupin-type phosphoglucose isomerases (Cupin-PGIs) constitute a novel metal-dependent PGI family representing a ...
|
|
6354
|
Cupin-type phosphoglucose isomerases (Cupin-PGIs) constitute a novel metal-dependent PGI family representing a ...
|
|
6355
|
Cupin-type phosphoglucose isomerases (Cupin-PGIs) constitute a novel metal-dependent PGI family representing a ...
|
|
6356
|
Cupin-type phosphoglucose isomerases (Cupin-PGIs) constitute a novel metal-dependent PGI family representing a ...
|
|
6357
|
Cupin-type phosphoglucose isomerases (Cupin-PGIs) constitute a novel metal-dependent PGI family representing a ...
|
|
6358
|
Cupin-type phosphoglucose isomerases (Cupin-PGIs) constitute a novel metal-dependent PGI family representing a ...
|
|
6359
|
Cupin-type phosphoglucose isomerases (Cupin-PGIs) constitute a novel metal-dependent PGI family representing a ...
|
|
6360
|
Site-directed mutagenesis of glutamate-190 in the hinge region of yeast 3-phosphoglycerate kinase: ...
|
|
6361
|
Site-directed mutagenesis of glutamate-190 in the hinge region of yeast 3-phosphoglycerate kinase: ...
|
|
6362
|
Site-directed mutagenesis of glutamate-190 in the hinge region of yeast 3-phosphoglycerate kinase: ...
|
|
6363
|
Site-directed mutagenesis of glutamate-190 in the hinge region of yeast 3-phosphoglycerate kinase: ...
|
|
6364
|
Site-directed mutagenesis of glutamate-190 in the hinge region of yeast 3-phosphoglycerate kinase: ...
|
|
6365
|
Site-directed mutagenesis of glutamate-190 in the hinge region of yeast 3-phosphoglycerate kinase: ...
|
|
6366
|
The phosphoglycerate kinases from Trypanosoma brucei. A comparison of the glycosomal and the cytosolic ...
|
|
6367
|
The phosphoglycerate kinases from Trypanosoma brucei. A comparison of the glycosomal and the cytosolic ...
|
|
6368
|
The phosphoglycerate kinases from Trypanosoma brucei. A comparison of the glycosomal and the cytosolic ...
|
|
6369
|
The phosphoglycerate kinases from Trypanosoma brucei. A comparison of the glycosomal and the cytosolic ...
|
|
6370
|
The phosphoglycerate kinases from Trypanosoma brucei. A comparison of the glycosomal and the cytosolic ...
|
|
6371
|
Stereospecificity of horseradish peroxidase
|
|
6372
|
Stereospecificity of horseradish peroxidase
|
|
6373
|
Stereospecificity of horseradish peroxidase
|
|
6374
|
Stereospecificity of horseradish peroxidase
|
|
6375
|
Stereospecificity of horseradish peroxidase
|
|
6376
|
Stereospecificity of horseradish peroxidase
|
|
6377
|
Stereospecificity of horseradish peroxidase
|
|
6378
|
Stereospecificity of horseradish peroxidase
|
|
6379
|
Stereospecificity of horseradish peroxidase
|
|
6380
|
Stereospecificity of horseradish peroxidase
|
|
6381
|
Stereospecificity of horseradish peroxidase
|
|
6382
|
Stereospecificity of horseradish peroxidase
|
|
6383
|
Stereospecificity of horseradish peroxidase
|
|
6384
|
Stereospecificity of horseradish peroxidase
|
|
6385
|
Stereospecificity of horseradish peroxidase
|
|
6386
|
Directed evolution of D-sialic acid aldolase to L-3-deoxy-manno-2-octulosonic acid (L-KDO) aldolase
|
|
6387
|
Directed evolution of D-sialic acid aldolase to L-3-deoxy-manno-2-octulosonic acid (L-KDO) aldolase
|
|
6388
|
Directed evolution of D-sialic acid aldolase to L-3-deoxy-manno-2-octulosonic acid (L-KDO) aldolase
|
|
6389
|
Directed evolution of D-sialic acid aldolase to L-3-deoxy-manno-2-octulosonic acid (L-KDO) aldolase
|
|
6390
|
Directed evolution of D-sialic acid aldolase to L-3-deoxy-manno-2-octulosonic acid (L-KDO) aldolase
|
|
6391
|
Directed evolution of D-sialic acid aldolase to L-3-deoxy-manno-2-octulosonic acid (L-KDO) aldolase
|
|
6392
|
Directed evolution of D-sialic acid aldolase to L-3-deoxy-manno-2-octulosonic acid (L-KDO) aldolase
|
|
6393
|
Directed evolution of D-sialic acid aldolase to L-3-deoxy-manno-2-octulosonic acid (L-KDO) aldolase
|
|
6394
|
Directed evolution of D-sialic acid aldolase to L-3-deoxy-manno-2-octulosonic acid (L-KDO) aldolase
|
|
6395
|
Directed evolution of D-sialic acid aldolase to L-3-deoxy-manno-2-octulosonic acid (L-KDO) aldolase
|
|
6396
|
Directed evolution of D-sialic acid aldolase to L-3-deoxy-manno-2-octulosonic acid (L-KDO) aldolase
|
|
6397
|
Directed evolution of D-sialic acid aldolase to L-3-deoxy-manno-2-octulosonic acid (L-KDO) aldolase
|
|
6398
|
Directed evolution of D-sialic acid aldolase to L-3-deoxy-manno-2-octulosonic acid (L-KDO) aldolase
|
|
6399
|
Directed evolution of D-sialic acid aldolase to L-3-deoxy-manno-2-octulosonic acid (L-KDO) aldolase
|
|
6400
|
Directed evolution of D-sialic acid aldolase to L-3-deoxy-manno-2-octulosonic acid (L-KDO) aldolase
|
|
6401
|
Directed evolution of D-sialic acid aldolase to L-3-deoxy-manno-2-octulosonic acid (L-KDO) aldolase
|
|
6402
|
Directed evolution of D-sialic acid aldolase to L-3-deoxy-manno-2-octulosonic acid (L-KDO) aldolase
|
|
6403
|
Directed evolution of D-sialic acid aldolase to L-3-deoxy-manno-2-octulosonic acid (L-KDO) aldolase
|
|
6404
|
Directed evolution of D-sialic acid aldolase to L-3-deoxy-manno-2-octulosonic acid (L-KDO) aldolase
|
|
6405
|
Directed evolution of D-sialic acid aldolase to L-3-deoxy-manno-2-octulosonic acid (L-KDO) aldolase
|
|
6406
|
Directed evolution of D-sialic acid aldolase to L-3-deoxy-manno-2-octulosonic acid (L-KDO) aldolase
|
|
6407
|
Directed evolution of D-sialic acid aldolase to L-3-deoxy-manno-2-octulosonic acid (L-KDO) aldolase
|
|
6408
|
Directed evolution of D-sialic acid aldolase to L-3-deoxy-manno-2-octulosonic acid (L-KDO) aldolase
|
|
6409
|
Physical and genetic interactions of cytosolic malate dehydrogenase with other gluconeogenic enzymes
|
|
6410
|
Physical and genetic interactions of cytosolic malate dehydrogenase with other gluconeogenic enzymes
|
|
6411
|
Physical and genetic interactions of cytosolic malate dehydrogenase with other gluconeogenic enzymes
|
|
6412
|
Physical and genetic interactions of cytosolic malate dehydrogenase with other gluconeogenic enzymes
|
|
6413
|
Physical and genetic interactions of cytosolic malate dehydrogenase with other gluconeogenic enzymes
|
|
6414
|
Physical and genetic interactions of cytosolic malate dehydrogenase with other gluconeogenic enzymes
|
|
6415
|
Physical and genetic interactions of cytosolic malate dehydrogenase with other gluconeogenic enzymes
|
|
6416
|
Physical and genetic interactions of cytosolic malate dehydrogenase with other gluconeogenic enzymes
|
|
6417
|
Comparison of control analysis data using different approaches: modelling and experiments with muscle extract
|
|
6418
|
Comparison of control analysis data using different approaches: modelling and experiments with muscle extract
|
|
6419
|
Comparison of control analysis data using different approaches: modelling and experiments with muscle extract
|
|
6420
|
Comparison of control analysis data using different approaches: modelling and experiments with muscle extract
|
|
6421
|
Probing the role of arginines and histidines in the catalytic function and activation of yeast ...
|
|
6422
|
Probing the role of arginines and histidines in the catalytic function and activation of yeast ...
|
|
6423
|
Probing the role of arginines and histidines in the catalytic function and activation of yeast ...
|
|
6424
|
Probing the role of arginines and histidines in the catalytic function and activation of yeast ...
|
|
6425
|
Probing the role of arginines and histidines in the catalytic function and activation of yeast ...
|
|
6426
|
Probing the role of arginines and histidines in the catalytic function and activation of yeast ...
|
|
6427
|
Probing the role of arginines and histidines in the catalytic function and activation of yeast ...
|
|
6428
|
Probing the role of arginines and histidines in the catalytic function and activation of yeast ...
|
|
6429
|
Probing the role of arginines and histidines in the catalytic function and activation of yeast ...
|
|
6430
|
Probing the role of arginines and histidines in the catalytic function and activation of yeast ...
|
|
6431
|
Probing the role of arginines and histidines in the catalytic function and activation of yeast ...
|
|
6432
|
Probing the role of arginines and histidines in the catalytic function and activation of yeast ...
|
|
6433
|
Probing the role of arginines and histidines in the catalytic function and activation of yeast ...
|
|
6434
|
Probing the role of arginines and histidines in the catalytic function and activation of yeast ...
|
|
6435
|
Probing the role of arginines and histidines in the catalytic function and activation of yeast ...
|
|
6436
|
Probing the role of arginines and histidines in the catalytic function and activation of yeast ...
|
|
6437
|
Probing the role of arginines and histidines in the catalytic function and activation of yeast ...
|
|
6438
|
Probing the role of arginines and histidines in the catalytic function and activation of yeast ...
|
|
6439
|
Probing the role of arginines and histidines in the catalytic function and activation of yeast ...
|
|
6440
|
Probing the role of arginines and histidines in the catalytic function and activation of yeast ...
|
|
6441
|
Characterization of three isoenzymes of rat alcohol dehydrogenase. Tissue distribution and physical and ...
|
|
6442
|
Characterization of three isoenzymes of rat alcohol dehydrogenase. Tissue distribution and physical and ...
|
|
6443
|
Characterization of three isoenzymes of rat alcohol dehydrogenase. Tissue distribution and physical and ...
|
|
6444
|
Characterization of three isoenzymes of rat alcohol dehydrogenase. Tissue distribution and physical and ...
|
|
6445
|
Characterization of three isoenzymes of rat alcohol dehydrogenase. Tissue distribution and physical and ...
|
|
6446
|
Characterization of three isoenzymes of rat alcohol dehydrogenase. Tissue distribution and physical and ...
|
|
6447
|
Characterization of three isoenzymes of rat alcohol dehydrogenase. Tissue distribution and physical and ...
|
|
6448
|
Characterization of three isoenzymes of rat alcohol dehydrogenase. Tissue distribution and physical and ...
|
|
6449
|
Characterization of three isoenzymes of rat alcohol dehydrogenase. Tissue distribution and physical and ...
|
|
6450
|
Characterization of three isoenzymes of rat alcohol dehydrogenase. Tissue distribution and physical and ...
|
|
6451
|
Characterization of three isoenzymes of rat alcohol dehydrogenase. Tissue distribution and physical and ...
|
|
6452
|
Characterization of three isoenzymes of rat alcohol dehydrogenase. Tissue distribution and physical and ...
|
|
6453
|
Characterization of three isoenzymes of rat alcohol dehydrogenase. Tissue distribution and physical and ...
|
|
6454
|
Characterization of three isoenzymes of rat alcohol dehydrogenase. Tissue distribution and physical and ...
|
|
6455
|
Characterization of three isoenzymes of rat alcohol dehydrogenase. Tissue distribution and physical and ...
|
|
6456
|
Characterization of three isoenzymes of rat alcohol dehydrogenase. Tissue distribution and physical and ...
|
|
6457
|
Characterization of three isoenzymes of rat alcohol dehydrogenase. Tissue distribution and physical and ...
|
|
6458
|
Characterization of three isoenzymes of rat alcohol dehydrogenase. Tissue distribution and physical and ...
|
|
6459
|
Characterization of three isoenzymes of rat alcohol dehydrogenase. Tissue distribution and physical and ...
|
|
6460
|
Characterization of three isoenzymes of rat alcohol dehydrogenase. Tissue distribution and physical and ...
|
|
6461
|
Characterization of three isoenzymes of rat alcohol dehydrogenase. Tissue distribution and physical and ...
|
|
6462
|
Characterization of three isoenzymes of rat alcohol dehydrogenase. Tissue distribution and physical and ...
|
|
6463
|
Characterization of three isoenzymes of rat alcohol dehydrogenase. Tissue distribution and physical and ...
|
|
6464
|
Characterization of three isoenzymes of rat alcohol dehydrogenase. Tissue distribution and physical and ...
|
|
6465
|
Characterization of three isoenzymes of rat alcohol dehydrogenase. Tissue distribution and physical and ...
|
|
6466
|
Characterization of three isoenzymes of rat alcohol dehydrogenase. Tissue distribution and physical and ...
|
|
6467
|
Characterization of three isoenzymes of rat alcohol dehydrogenase. Tissue distribution and physical and ...
|
|
6468
|
Characterization of three isoenzymes of rat alcohol dehydrogenase. Tissue distribution and physical and ...
|
|
6469
|
Characterization of three isoenzymes of rat alcohol dehydrogenase. Tissue distribution and physical and ...
|
|
6470
|
Characterization of three isoenzymes of rat alcohol dehydrogenase. Tissue distribution and physical and ...
|
|
6471
|
Characterization of three isoenzymes of rat alcohol dehydrogenase. Tissue distribution and physical and ...
|
|
6472
|
Characterization of three isoenzymes of rat alcohol dehydrogenase. Tissue distribution and physical and ...
|
|
6473
|
Characterization of three isoenzymes of rat alcohol dehydrogenase. Tissue distribution and physical and ...
|
|
6474
|
Characterization of three isoenzymes of rat alcohol dehydrogenase. Tissue distribution and physical and ...
|
|
6475
|
Characterization of three isoenzymes of rat alcohol dehydrogenase. Tissue distribution and physical and ...
|
|
6476
|
Characterization of three isoenzymes of rat alcohol dehydrogenase. Tissue distribution and physical and ...
|
|
6477
|
Characterization of three isoenzymes of rat alcohol dehydrogenase. Tissue distribution and physical and ...
|
|
6478
|
Characterization of three isoenzymes of rat alcohol dehydrogenase. Tissue distribution and physical and ...
|
|
6479
|
Characterization of three isoenzymes of rat alcohol dehydrogenase. Tissue distribution and physical and ...
|
|
6480
|
Characterization of three isoenzymes of rat alcohol dehydrogenase. Tissue distribution and physical and ...
|
|
6481
|
Characterization of three isoenzymes of rat alcohol dehydrogenase. Tissue distribution and physical and ...
|
|
6482
|
Characterization of three isoenzymes of rat alcohol dehydrogenase. Tissue distribution and physical and ...
|
|
6483
|
Characterization of three isoenzymes of rat alcohol dehydrogenase. Tissue distribution and physical and ...
|
|
6484
|
Characterization of three isoenzymes of rat alcohol dehydrogenase. Tissue distribution and physical and ...
|
|
6485
|
Characterization of three isoenzymes of rat alcohol dehydrogenase. Tissue distribution and physical and ...
|
|
6486
|
Characterization of three isoenzymes of rat alcohol dehydrogenase. Tissue distribution and physical and ...
|
|
6487
|
Characterization of three isoenzymes of rat alcohol dehydrogenase. Tissue distribution and physical and ...
|
|
6488
|
Characterization of three isoenzymes of rat alcohol dehydrogenase. Tissue distribution and physical and ...
|
|
6489
|
Characterization of three isoenzymes of rat alcohol dehydrogenase. Tissue distribution and physical and ...
|
|
6490
|
Characterization of three isoenzymes of rat alcohol dehydrogenase. Tissue distribution and physical and ...
|
|
6491
|
Characterization of three isoenzymes of rat alcohol dehydrogenase. Tissue distribution and physical and ...
|
|
6492
|
Phylogenetic relationships and biochemical properties of the duplicated cytosolic and mitochondrial isoforms ...
|
|
6493
|
Phylogenetic relationships and biochemical properties of the duplicated cytosolic and mitochondrial isoforms ...
|
|
6494
|
Phylogenetic relationships and biochemical properties of the duplicated cytosolic and mitochondrial isoforms ...
|
|
6495
|
Phylogenetic relationships and biochemical properties of the duplicated cytosolic and mitochondrial isoforms ...
|
|
6496
|
Phylogenetic relationships and biochemical properties of the duplicated cytosolic and mitochondrial isoforms ...
|
|
6497
|
Phylogenetic relationships and biochemical properties of the duplicated cytosolic and mitochondrial isoforms ...
|
|
6498
|
Phylogenetic relationships and biochemical properties of the duplicated cytosolic and mitochondrial isoforms ...
|
|
6499
|
Phylogenetic relationships and biochemical properties of the duplicated cytosolic and mitochondrial isoforms ...
|
|
6500
|
Phylogenetic relationships and biochemical properties of the duplicated cytosolic and mitochondrial isoforms ...
|
|
6501
|
Phylogenetic relationships and biochemical properties of the duplicated cytosolic and mitochondrial isoforms ...
|
|
6502
|
Phylogenetic relationships and biochemical properties of the duplicated cytosolic and mitochondrial isoforms ...
|
|
6503
|
Phylogenetic relationships and biochemical properties of the duplicated cytosolic and mitochondrial isoforms ...
|
|
6504
|
Xenopus laevis CYP17 regulates androgen biosynthesis independent of the cofactor cytochrome b5
|
|
6505
|
Xenopus laevis CYP17 regulates androgen biosynthesis independent of the cofactor cytochrome b5
|
|
6506
|
Xenopus laevis CYP17 regulates androgen biosynthesis independent of the cofactor cytochrome b5
|
|
6507
|
Xenopus laevis CYP17 regulates androgen biosynthesis independent of the cofactor cytochrome b5
|
|
6508
|
Xenopus laevis CYP17 regulates androgen biosynthesis independent of the cofactor cytochrome b5
|
|
6509
|
Xenopus laevis CYP17 regulates androgen biosynthesis independent of the cofactor cytochrome b5
|
|
6510
|
Xenopus laevis CYP17 regulates androgen biosynthesis independent of the cofactor cytochrome b5
|
|
6511
|
Xenopus laevis CYP17 regulates androgen biosynthesis independent of the cofactor cytochrome b5
|
|
6512
|
Xenopus laevis CYP17 regulates androgen biosynthesis independent of the cofactor cytochrome b5
|
|
6513
|
Xenopus laevis CYP17 regulates androgen biosynthesis independent of the cofactor cytochrome b5
|
|
6514
|
Xenopus laevis CYP17 regulates androgen biosynthesis independent of the cofactor cytochrome b5
|
|
6515
|
Xenopus laevis CYP17 regulates androgen biosynthesis independent of the cofactor cytochrome b5
|
|
6516
|
Xenopus laevis CYP17 regulates androgen biosynthesis independent of the cofactor cytochrome b5
|
|
6517
|
Xenopus laevis CYP17 regulates androgen biosynthesis independent of the cofactor cytochrome b5
|
|
6518
|
Xenopus laevis CYP17 regulates androgen biosynthesis independent of the cofactor cytochrome b5
|
|
6519
|
Xenopus laevis CYP17 regulates androgen biosynthesis independent of the cofactor cytochrome b5
|
|
6520
|
Xenopus laevis CYP17 regulates androgen biosynthesis independent of the cofactor cytochrome b5
|
|
6521
|
Xenopus laevis CYP17 regulates androgen biosynthesis independent of the cofactor cytochrome b5
|
|
6522
|
Xenopus laevis CYP17 regulates androgen biosynthesis independent of the cofactor cytochrome b5
|
|
6523
|
Xenopus laevis CYP17 regulates androgen biosynthesis independent of the cofactor cytochrome b5
|
|
6524
|
Xenopus laevis CYP17 regulates androgen biosynthesis independent of the cofactor cytochrome b5
|
|
6525
|
Xenopus laevis CYP17 regulates androgen biosynthesis independent of the cofactor cytochrome b5
|
|
6526
|
Xenopus laevis CYP17 regulates androgen biosynthesis independent of the cofactor cytochrome b5
|
|
6527
|
Xenopus laevis CYP17 regulates androgen biosynthesis independent of the cofactor cytochrome b5
|
|
6528
|
Xenopus laevis CYP17 regulates androgen biosynthesis independent of the cofactor cytochrome b5
|
|
6529
|
Xenopus laevis CYP17 regulates androgen biosynthesis independent of the cofactor cytochrome b5
|
|
6530
|
Xenopus laevis CYP17 regulates androgen biosynthesis independent of the cofactor cytochrome b5
|
|
6531
|
Functional and structural analysis of catalase oxidized by singlet oxygen
|
|
6532
|
Functional and structural analysis of catalase oxidized by singlet oxygen
|
|
6533
|
Functional and structural analysis of catalase oxidized by singlet oxygen
|
|
6534
|
Functional and structural analysis of catalase oxidized by singlet oxygen
|
|
6535
|
Functional and structural analysis of catalase oxidized by singlet oxygen
|
|
6536
|
Functional and structural analysis of catalase oxidized by singlet oxygen
|
|
6537
|
Functional and structural analysis of catalase oxidized by singlet oxygen
|
|
6538
|
Functional and structural analysis of catalase oxidized by singlet oxygen
|
|
6539
|
Functional and structural analysis of catalase oxidized by singlet oxygen
|
|
6540
|
Functional and structural analysis of catalase oxidized by singlet oxygen
|
|
6541
|
Isotope and elemental effects indicate a rate-limiting methyl transfer as the initial step in the reaction ...
|
|
6542
|
Isotope and elemental effects indicate a rate-limiting methyl transfer as the initial step in the reaction ...
|
|
6543
|
Isotope and elemental effects indicate a rate-limiting methyl transfer as the initial step in the reaction ...
|
|
6544
|
Isotope and elemental effects indicate a rate-limiting methyl transfer as the initial step in the reaction ...
|
|
6545
|
Isotope and elemental effects indicate a rate-limiting methyl transfer as the initial step in the reaction ...
|
|
6546
|
The synthesis of mannose 1-phosphate in brain
|
|
6547
|
The synthesis of mannose 1-phosphate in brain
|
|
6548
|
The synthesis of mannose 1-phosphate in brain
|
|
6549
|
Isocitrate dehydrogenase of Plasmodium falciparum
|
|
6550
|
Isocitrate dehydrogenase of Plasmodium falciparum
|
|
6551
|
Isocitrate dehydrogenase of Plasmodium falciparum
|
|
6552
|
Isocitrate dehydrogenase of Plasmodium falciparum
|
|
6569
|
Engineering a domain-locking disulfide into a bacterial malate dehydrogenase produces a redox-sensitive enzyme
|
|
6570
|
Engineering a domain-locking disulfide into a bacterial malate dehydrogenase produces a redox-sensitive enzyme
|
|
6571
|
Engineering a domain-locking disulfide into a bacterial malate dehydrogenase produces a redox-sensitive enzyme
|
|
6572
|
Engineering a domain-locking disulfide into a bacterial malate dehydrogenase produces a redox-sensitive enzyme
|
|
6573
|
Engineering a domain-locking disulfide into a bacterial malate dehydrogenase produces a redox-sensitive enzyme
|
|
6574
|
Engineering a domain-locking disulfide into a bacterial malate dehydrogenase produces a redox-sensitive enzyme
|
|
6575
|
Engineering a domain-locking disulfide into a bacterial malate dehydrogenase produces a redox-sensitive enzyme
|
|
6576
|
Engineering a domain-locking disulfide into a bacterial malate dehydrogenase produces a redox-sensitive enzyme
|
|
6577
|
Engineering a domain-locking disulfide into a bacterial malate dehydrogenase produces a redox-sensitive enzyme
|
|
6578
|
Engineering a domain-locking disulfide into a bacterial malate dehydrogenase produces a redox-sensitive enzyme
|
|
6579
|
Engineering a domain-locking disulfide into a bacterial malate dehydrogenase produces a redox-sensitive enzyme
|
|
6580
|
Engineering a domain-locking disulfide into a bacterial malate dehydrogenase produces a redox-sensitive enzyme
|
|
6581
|
Engineering a domain-locking disulfide into a bacterial malate dehydrogenase produces a redox-sensitive enzyme
|
|
6582
|
Engineering a domain-locking disulfide into a bacterial malate dehydrogenase produces a redox-sensitive enzyme
|
|
6583
|
Probing the catalytic center of porcine aminoacylase 1 by site-directed mutagenesis, homology modeling and ...
|
|
6584
|
Probing the catalytic center of porcine aminoacylase 1 by site-directed mutagenesis, homology modeling and ...
|
|
6585
|
Probing the catalytic center of porcine aminoacylase 1 by site-directed mutagenesis, homology modeling and ...
|
|
6586
|
Probing the catalytic center of porcine aminoacylase 1 by site-directed mutagenesis, homology modeling and ...
|
|
6587
|
Probing the catalytic center of porcine aminoacylase 1 by site-directed mutagenesis, homology modeling and ...
|
|
6588
|
Probing the catalytic center of porcine aminoacylase 1 by site-directed mutagenesis, homology modeling and ...
|
|
6589
|
Probing the catalytic center of porcine aminoacylase 1 by site-directed mutagenesis, homology modeling and ...
|
|
6590
|
Probing the catalytic center of porcine aminoacylase 1 by site-directed mutagenesis, homology modeling and ...
|
|
6591
|
Probing the catalytic center of porcine aminoacylase 1 by site-directed mutagenesis, homology modeling and ...
|
|
6592
|
Probing the catalytic center of porcine aminoacylase 1 by site-directed mutagenesis, homology modeling and ...
|
|
6593
|
Probing the catalytic center of porcine aminoacylase 1 by site-directed mutagenesis, homology modeling and ...
|
|
6594
|
Probing the catalytic center of porcine aminoacylase 1 by site-directed mutagenesis, homology modeling and ...
|
|
6595
|
Probing the catalytic center of porcine aminoacylase 1 by site-directed mutagenesis, homology modeling and ...
|
|
6596
|
The isolation and characterization of the aspartate transcarbamylase domain of the multifunctional protein, ...
|
|
6597
|
The isolation and characterization of the aspartate transcarbamylase domain of the multifunctional protein, ...
|
|
6598
|
The isolation and characterization of the aspartate transcarbamylase domain of the multifunctional protein, ...
|
|
6599
|
The isolation and characterization of the aspartate transcarbamylase domain of the multifunctional protein, ...
|
|
6600
|
In vitro mutagenesis studies at the arginine residues of adenylate kinase. A revised binding site for AMP in ...
|
|
6601
|
In vitro mutagenesis studies at the arginine residues of adenylate kinase. A revised binding site for AMP in ...
|
|
6602
|
In vitro mutagenesis studies at the arginine residues of adenylate kinase. A revised binding site for AMP in ...
|
|
6603
|
In vitro mutagenesis studies at the arginine residues of adenylate kinase. A revised binding site for AMP in ...
|
|
6604
|
In vitro mutagenesis studies at the arginine residues of adenylate kinase. A revised binding site for AMP in ...
|
|
6605
|
In vitro mutagenesis studies at the arginine residues of adenylate kinase. A revised binding site for AMP in ...
|
|
6606
|
In vitro mutagenesis studies at the arginine residues of adenylate kinase. A revised binding site for AMP in ...
|
|
6607
|
In vitro mutagenesis studies at the arginine residues of adenylate kinase. A revised binding site for AMP in ...
|
|
6608
|
In vitro mutagenesis studies at the arginine residues of adenylate kinase. A revised binding site for AMP in ...
|
|
6609
|
In vitro mutagenesis studies at the arginine residues of adenylate kinase. A revised binding site for AMP in ...
|
|
6610
|
In vitro mutagenesis studies at the arginine residues of adenylate kinase. A revised binding site for AMP in ...
|
|
6611
|
In vitro mutagenesis studies at the arginine residues of adenylate kinase. A revised binding site for AMP in ...
|
|
6612
|
In vitro mutagenesis studies at the arginine residues of adenylate kinase. A revised binding site for AMP in ...
|
|
6613
|
In vitro mutagenesis studies at the arginine residues of adenylate kinase. A revised binding site for AMP in ...
|
|
6614
|
The binding mechanism of the yeast F1-ATPase inhibitory peptide: role of catalytic intermediates and enzyme ...
|
|
6615
|
The binding mechanism of the yeast F1-ATPase inhibitory peptide: role of catalytic intermediates and enzyme ...
|
|
6616
|
The binding mechanism of the yeast F1-ATPase inhibitory peptide: role of catalytic intermediates and enzyme ...
|
|
6617
|
The conserved cis-Pro39 residue plays a crucial role in the proper positioning of the catalytic base Asp38 in ...
|
|
6618
|
The conserved cis-Pro39 residue plays a crucial role in the proper positioning of the catalytic base Asp38 in ...
|
|
6619
|
The conserved cis-Pro39 residue plays a crucial role in the proper positioning of the catalytic base Asp38 in ...
|
|
6620
|
The conserved cis-Pro39 residue plays a crucial role in the proper positioning of the catalytic base Asp38 in ...
|
|
6621
|
The conserved cis-Pro39 residue plays a crucial role in the proper positioning of the catalytic base Asp38 in ...
|
|
6622
|
The conserved cis-Pro39 residue plays a crucial role in the proper positioning of the catalytic base Asp38 in ...
|
|
6623
|
Use of cultured lymphoblastoid cells for the study of abnormal enzymes: molecular abnormality of a ...
|
|
6624
|
Use of cultured lymphoblastoid cells for the study of abnormal enzymes: molecular abnormality of a ...
|
|
6625
|
Use of cultured lymphoblastoid cells for the study of abnormal enzymes: molecular abnormality of a ...
|
|
6626
|
Use of cultured lymphoblastoid cells for the study of abnormal enzymes: molecular abnormality of a ...
|
|
6627
|
Use of cultured lymphoblastoid cells for the study of abnormal enzymes: molecular abnormality of a ...
|
|
6628
|
Use of cultured lymphoblastoid cells for the study of abnormal enzymes: molecular abnormality of a ...
|
|
6629
|
Expression of active octameric chicken cardiac mitochondrial creatine kinase in Escherichia coli
|
|
6630
|
Expression of active octameric chicken cardiac mitochondrial creatine kinase in Escherichia coli
|
|
6631
|
Expression of active octameric chicken cardiac mitochondrial creatine kinase in Escherichia coli
|
|
6632
|
Expression of active octameric chicken cardiac mitochondrial creatine kinase in Escherichia coli
|
|
6633
|
Oxidation of resveratrol catalyzed by soybean lipoxygenase
|
|
6634
|
Oxidation of resveratrol catalyzed by soybean lipoxygenase
|
|
6635
|
Oxidation of resveratrol catalyzed by soybean lipoxygenase
|
|
6636
|
Oxidation of resveratrol catalyzed by soybean lipoxygenase
|
|
6637
|
Oxidation of resveratrol catalyzed by soybean lipoxygenase
|
|
6638
|
Oxidation of resveratrol catalyzed by soybean lipoxygenase
|
|
6639
|
Oxidation of resveratrol catalyzed by soybean lipoxygenase
|
|
6640
|
Oxidation of resveratrol catalyzed by soybean lipoxygenase
|
|
6641
|
Oxidation of resveratrol catalyzed by soybean lipoxygenase
|
|
6642
|
Oxidation of resveratrol catalyzed by soybean lipoxygenase
|
|
6643
|
Oxidation of resveratrol catalyzed by soybean lipoxygenase
|
|
6644
|
Oxidation of resveratrol catalyzed by soybean lipoxygenase
|
|
6645
|
Oxidation of resveratrol catalyzed by soybean lipoxygenase
|
|
6646
|
Transient and equilibrium kinetic studies on yeast 3-phosphoglycerate kinase. Evidence that an intermediate ...
|
|
6647
|
Transient and equilibrium kinetic studies on yeast 3-phosphoglycerate kinase. Evidence that an intermediate ...
|
|
6648
|
Transient and equilibrium kinetic studies on yeast 3-phosphoglycerate kinase. Evidence that an intermediate ...
|
|
6649
|
Transient and equilibrium kinetic studies on yeast 3-phosphoglycerate kinase. Evidence that an intermediate ...
|
|
6650
|
Transient and equilibrium kinetic studies on yeast 3-phosphoglycerate kinase. Evidence that an intermediate ...
|
|
6651
|
Transient and equilibrium kinetic studies on yeast 3-phosphoglycerate kinase. Evidence that an intermediate ...
|
|
6652
|
Transient and equilibrium kinetic studies on yeast 3-phosphoglycerate kinase. Evidence that an intermediate ...
|
|
6653
|
Transient and equilibrium kinetic studies on yeast 3-phosphoglycerate kinase. Evidence that an intermediate ...
|
|
6654
|
Synthesis and properties of a conformationally restricted spin-labeled analog of ATP and its interaction with ...
|
|
6655
|
Synthesis and properties of a conformationally restricted spin-labeled analog of ATP and its interaction with ...
|
|
6656
|
Synthesis and properties of a conformationally restricted spin-labeled analog of ATP and its interaction with ...
|
|
6657
|
Synthesis and properties of a conformationally restricted spin-labeled analog of ATP and its interaction with ...
|
|
6658
|
Evidence that 1,3-bisphosphoglycerate dissociation from phosphoglycerate kinase is an intrinsically rapid ...
|
|
6659
|
The binding of beta- and gamma-cyclodextrins to glycogen phosphorylase b: kinetic and crystallographic studies
|
|
6660
|
The binding of beta- and gamma-cyclodextrins to glycogen phosphorylase b: kinetic and crystallographic studies
|
|
6661
|
The binding of beta- and gamma-cyclodextrins to glycogen phosphorylase b: kinetic and crystallographic studies
|
|
6662
|
Altering substrate specificity of phosphatidylcholine-preferring phospholipase C of Bacillus cereus by random ...
|
|
6663
|
Altering substrate specificity of phosphatidylcholine-preferring phospholipase C of Bacillus cereus by random ...
|
|
6664
|
Altering substrate specificity of phosphatidylcholine-preferring phospholipase C of Bacillus cereus by random ...
|
|
6665
|
Altering substrate specificity of phosphatidylcholine-preferring phospholipase C of Bacillus cereus by random ...
|
|
6666
|
Altering substrate specificity of phosphatidylcholine-preferring phospholipase C of Bacillus cereus by random ...
|
|
6667
|
Altering substrate specificity of phosphatidylcholine-preferring phospholipase C of Bacillus cereus by random ...
|
|
6668
|
Altering substrate specificity of phosphatidylcholine-preferring phospholipase C of Bacillus cereus by random ...
|
|
6669
|
Altering substrate specificity of phosphatidylcholine-preferring phospholipase C of Bacillus cereus by random ...
|
|
6670
|
Altering substrate specificity of phosphatidylcholine-preferring phospholipase C of Bacillus cereus by random ...
|
|
6671
|
Altering substrate specificity of phosphatidylcholine-preferring phospholipase C of Bacillus cereus by random ...
|
|
6672
|
Altering substrate specificity of phosphatidylcholine-preferring phospholipase C of Bacillus cereus by random ...
|
|
6673
|
Altering substrate specificity of phosphatidylcholine-preferring phospholipase C of Bacillus cereus by random ...
|
|
6674
|
Altering substrate specificity of phosphatidylcholine-preferring phospholipase C of Bacillus cereus by random ...
|
|
6675
|
Altering substrate specificity of phosphatidylcholine-preferring phospholipase C of Bacillus cereus by random ...
|
|
6676
|
Altering substrate specificity of phosphatidylcholine-preferring phospholipase C of Bacillus cereus by random ...
|
|
6677
|
Altering substrate specificity of phosphatidylcholine-preferring phospholipase C of Bacillus cereus by random ...
|
|
6678
|
Altering substrate specificity of phosphatidylcholine-preferring phospholipase C of Bacillus cereus by random ...
|
|
6679
|
Altering substrate specificity of phosphatidylcholine-preferring phospholipase C of Bacillus cereus by random ...
|
|
6680
|
Altering substrate specificity of phosphatidylcholine-preferring phospholipase C of Bacillus cereus by random ...
|
|
6681
|
Altering substrate specificity of phosphatidylcholine-preferring phospholipase C of Bacillus cereus by random ...
|
|
6682
|
Altering substrate specificity of phosphatidylcholine-preferring phospholipase C of Bacillus cereus by random ...
|
|
6683
|
Altering substrate specificity of phosphatidylcholine-preferring phospholipase C of Bacillus cereus by random ...
|
|
6684
|
Altering substrate specificity of phosphatidylcholine-preferring phospholipase C of Bacillus cereus by random ...
|
|
6685
|
Altering substrate specificity of phosphatidylcholine-preferring phospholipase C of Bacillus cereus by random ...
|
|
6686
|
Altering substrate specificity of phosphatidylcholine-preferring phospholipase C of Bacillus cereus by random ...
|
|
6687
|
Altering substrate specificity of phosphatidylcholine-preferring phospholipase C of Bacillus cereus by random ...
|
|
6688
|
Altering substrate specificity of phosphatidylcholine-preferring phospholipase C of Bacillus cereus by random ...
|
|
6689
|
Altering substrate specificity of phosphatidylcholine-preferring phospholipase C of Bacillus cereus by random ...
|
|
6690
|
Altering substrate specificity of phosphatidylcholine-preferring phospholipase C of Bacillus cereus by random ...
|
|
6691
|
Altering substrate specificity of phosphatidylcholine-preferring phospholipase C of Bacillus cereus by random ...
|
|
6692
|
Altering substrate specificity of phosphatidylcholine-preferring phospholipase C of Bacillus cereus by random ...
|
|
6693
|
Altering substrate specificity of phosphatidylcholine-preferring phospholipase C of Bacillus cereus by random ...
|
|
6694
|
Altering substrate specificity of phosphatidylcholine-preferring phospholipase C of Bacillus cereus by random ...
|
|
6695
|
Altering substrate specificity of phosphatidylcholine-preferring phospholipase C of Bacillus cereus by random ...
|
|
6696
|
Altering substrate specificity of phosphatidylcholine-preferring phospholipase C of Bacillus cereus by random ...
|
|
6697
|
Altering substrate specificity of phosphatidylcholine-preferring phospholipase C of Bacillus cereus by random ...
|
|
6698
|
Altering substrate specificity of phosphatidylcholine-preferring phospholipase C of Bacillus cereus by random ...
|
|
6699
|
Altering substrate specificity of phosphatidylcholine-preferring phospholipase C of Bacillus cereus by random ...
|
|
6700
|
Altering substrate specificity of phosphatidylcholine-preferring phospholipase C of Bacillus cereus by random ...
|
|
6701
|
Altering substrate specificity of phosphatidylcholine-preferring phospholipase C of Bacillus cereus by random ...
|
|
6702
|
Altering substrate specificity of phosphatidylcholine-preferring phospholipase C of Bacillus cereus by random ...
|
|
6703
|
Altering substrate specificity of phosphatidylcholine-preferring phospholipase C of Bacillus cereus by random ...
|
|
6704
|
Altering substrate specificity of phosphatidylcholine-preferring phospholipase C of Bacillus cereus by random ...
|
|
6705
|
Altering substrate specificity of phosphatidylcholine-preferring phospholipase C of Bacillus cereus by random ...
|
|
6706
|
Altering substrate specificity of phosphatidylcholine-preferring phospholipase C of Bacillus cereus by random ...
|
|
6707
|
Altering substrate specificity of phosphatidylcholine-preferring phospholipase C of Bacillus cereus by random ...
|
|
6708
|
Altering substrate specificity of phosphatidylcholine-preferring phospholipase C of Bacillus cereus by random ...
|
|
6709
|
Altering substrate specificity of phosphatidylcholine-preferring phospholipase C of Bacillus cereus by random ...
|
|
6710
|
Altering substrate specificity of phosphatidylcholine-preferring phospholipase C of Bacillus cereus by random ...
|
|
6711
|
Altering substrate specificity of phosphatidylcholine-preferring phospholipase C of Bacillus cereus by random ...
|
|
6712
|
Altering substrate specificity of phosphatidylcholine-preferring phospholipase C of Bacillus cereus by random ...
|
|
6713
|
Altering substrate specificity of phosphatidylcholine-preferring phospholipase C of Bacillus cereus by random ...
|
|
6714
|
Altering substrate specificity of phosphatidylcholine-preferring phospholipase C of Bacillus cereus by random ...
|
|
6715
|
Altering substrate specificity of phosphatidylcholine-preferring phospholipase C of Bacillus cereus by random ...
|
|
6716
|
Altering substrate specificity of phosphatidylcholine-preferring phospholipase C of Bacillus cereus by random ...
|
|
6717
|
Altering substrate specificity of phosphatidylcholine-preferring phospholipase C of Bacillus cereus by random ...
|
|
6718
|
Altering substrate specificity of phosphatidylcholine-preferring phospholipase C of Bacillus cereus by random ...
|
|
6719
|
Phosphoglucoisomerase-catalyzed interconversion of hexose phosphates: a model for the interconversion of ...
|
|
6720
|
Phosphoglucoisomerase-catalyzed interconversion of hexose phosphates: a model for the interconversion of ...
|
|
6721
|
Phosphoglucoisomerase-catalyzed interconversion of hexose phosphates: a model for the interconversion of ...
|
|
6722
|
Phosphoglucoisomerase-catalyzed interconversion of hexose phosphates: a model for the interconversion of ...
|
|
6723
|
Family 39 alpha-l-iduronidases and beta-D-xylosidases react through similar glycosyl-enzyme intermediates: ...
|
|
6724
|
Family 39 alpha-l-iduronidases and beta-D-xylosidases react through similar glycosyl-enzyme intermediates: ...
|
|
6725
|
Family 39 alpha-l-iduronidases and beta-D-xylosidases react through similar glycosyl-enzyme intermediates: ...
|
|
6726
|
Purification and characterization of phosphoglucose isomerase allozymes from Daphnia magna
|
|
6727
|
Purification and characterization of phosphoglucose isomerase allozymes from Daphnia magna
|
|
6728
|
Purification and characterization of phosphoglucose isomerase allozymes from Daphnia magna
|
|
6729
|
Cytosolic mercaptopyruvate sulfurtransferase is evolutionarily related to mitochondrial rhodanese. Striking ...
|
|
6730
|
Cytosolic mercaptopyruvate sulfurtransferase is evolutionarily related to mitochondrial rhodanese. Striking ...
|
|
6731
|
Cytosolic mercaptopyruvate sulfurtransferase is evolutionarily related to mitochondrial rhodanese. Striking ...
|
|
6732
|
Cytosolic mercaptopyruvate sulfurtransferase is evolutionarily related to mitochondrial rhodanese. Striking ...
|
|
6733
|
Cytosolic mercaptopyruvate sulfurtransferase is evolutionarily related to mitochondrial rhodanese. Striking ...
|
|
6734
|
Cytosolic mercaptopyruvate sulfurtransferase is evolutionarily related to mitochondrial rhodanese. Striking ...
|
|
6735
|
Cytosolic mercaptopyruvate sulfurtransferase is evolutionarily related to mitochondrial rhodanese. Striking ...
|
|
6736
|
Cytosolic mercaptopyruvate sulfurtransferase is evolutionarily related to mitochondrial rhodanese. Striking ...
|
|
6737
|
Cytosolic mercaptopyruvate sulfurtransferase is evolutionarily related to mitochondrial rhodanese. Striking ...
|
|
6738
|
Cytosolic mercaptopyruvate sulfurtransferase is evolutionarily related to mitochondrial rhodanese. Striking ...
|
|
6739
|
Role of amino acid residues in th active site of rat liver Mercaptopyruvate Sulfurtransferase- cDNA cloning, ...
|
|
6740
|
Role of amino acid residues in th active site of rat liver Mercaptopyruvate Sulfurtransferase- cDNA cloning, ...
|
|
6741
|
Role of amino acid residues in th active site of rat liver Mercaptopyruvate Sulfurtransferase- cDNA cloning, ...
|
|
6742
|
Role of amino acid residues in th active site of rat liver Mercaptopyruvate Sulfurtransferase- cDNA cloning, ...
|
|
6743
|
Role of amino acid residues in th active site of rat liver Mercaptopyruvate Sulfurtransferase- cDNA cloning, ...
|
|
6744
|
Role of amino acid residues in th active site of rat liver Mercaptopyruvate Sulfurtransferase- cDNA cloning, ...
|
|
6745
|
Role of amino acid residues in th active site of rat liver Mercaptopyruvate Sulfurtransferase- cDNA cloning, ...
|
|
6746
|
Role of amino acid residues in th active site of rat liver Mercaptopyruvate Sulfurtransferase- cDNA cloning, ...
|
|
6747
|
Role of amino acid residues in th active site of rat liver Mercaptopyruvate Sulfurtransferase- cDNA cloning, ...
|
|
6748
|
Role of amino acid residues in th active site of rat liver Mercaptopyruvate Sulfurtransferase- cDNA cloning, ...
|
|
6749
|
Role of amino acid residues in th active site of rat liver Mercaptopyruvate Sulfurtransferase- cDNA cloning, ...
|
|
6750
|
Role of amino acid residues in th active site of rat liver Mercaptopyruvate Sulfurtransferase- cDNA cloning, ...
|
|
6751
|
Comparative enzymology of the glyceraldehyde 3-phosphate dehydrogenases from pisum sativum
|
|
6752
|
Mechanistic roles of Ser-114, Tyr-155, and Lys-159 in 3alpha-hydroxysteroid dehydrogenase/carbonyl reductase ...
|
|
6753
|
Mechanistic roles of Ser-114, Tyr-155, and Lys-159 in 3alpha-hydroxysteroid dehydrogenase/carbonyl reductase ...
|
|
6754
|
Mechanistic roles of Ser-114, Tyr-155, and Lys-159 in 3alpha-hydroxysteroid dehydrogenase/carbonyl reductase ...
|
|
6755
|
Mechanistic roles of Ser-114, Tyr-155, and Lys-159 in 3alpha-hydroxysteroid dehydrogenase/carbonyl reductase ...
|
|
6756
|
Mechanistic roles of Ser-114, Tyr-155, and Lys-159 in 3alpha-hydroxysteroid dehydrogenase/carbonyl reductase ...
|
|
6757
|
Mechanistic roles of Ser-114, Tyr-155, and Lys-159 in 3alpha-hydroxysteroid dehydrogenase/carbonyl reductase ...
|
|
6758
|
Mechanistic roles of Ser-114, Tyr-155, and Lys-159 in 3alpha-hydroxysteroid dehydrogenase/carbonyl reductase ...
|
|
6759
|
Mechanistic roles of Ser-114, Tyr-155, and Lys-159 in 3alpha-hydroxysteroid dehydrogenase/carbonyl reductase ...
|
|
6760
|
Mechanistic roles of Ser-114, Tyr-155, and Lys-159 in 3alpha-hydroxysteroid dehydrogenase/carbonyl reductase ...
|
|
6761
|
Mechanistic roles of Ser-114, Tyr-155, and Lys-159 in 3alpha-hydroxysteroid dehydrogenase/carbonyl reductase ...
|
|
6762
|
Mechanistic roles of Ser-114, Tyr-155, and Lys-159 in 3alpha-hydroxysteroid dehydrogenase/carbonyl reductase ...
|
|
6763
|
Compartmentation and kinetics of urea cycle enzymes in porcine enterocytes
|
|
6764
|
Compartmentation and kinetics of urea cycle enzymes in porcine enterocytes
|
|
6765
|
Compartmentation and kinetics of urea cycle enzymes in porcine enterocytes
|
|
6766
|
Compartmentation and kinetics of urea cycle enzymes in porcine enterocytes
|
|
6767
|
Compartmentation and kinetics of urea cycle enzymes in porcine enterocytes
|
|
6768
|
Compartmentation and kinetics of urea cycle enzymes in porcine enterocytes
|
|
6769
|
Compartmentation and kinetics of urea cycle enzymes in porcine enterocytes
|
|
6770
|
Compartmentation and kinetics of urea cycle enzymes in porcine enterocytes
|
|
6771
|
Compartmentation and kinetics of urea cycle enzymes in porcine enterocytes
|
|
6772
|
Compartmentation and kinetics of urea cycle enzymes in porcine enterocytes
|
|
6773
|
Compartmentation and kinetics of urea cycle enzymes in porcine enterocytes
|
|
6774
|
Compartmentation and kinetics of urea cycle enzymes in porcine enterocytes
|
|
6775
|
Compartmentation and kinetics of urea cycle enzymes in porcine enterocytes
|
|
6776
|
Compartmentation and kinetics of urea cycle enzymes in porcine enterocytes
|
|
6777
|
Compartmentation and kinetics of urea cycle enzymes in porcine enterocytes
|
|
6778
|
Compartmentation and kinetics of urea cycle enzymes in porcine enterocytes
|
|
6779
|
Compartmentation and kinetics of urea cycle enzymes in porcine enterocytes
|
|
6780
|
Compartmentation and kinetics of urea cycle enzymes in porcine enterocytes
|
|
6781
|
Compartmentation and kinetics of urea cycle enzymes in porcine enterocytes
|
|
6782
|
Compartmentation and kinetics of urea cycle enzymes in porcine enterocytes
|
|
6783
|
Compartmentation and kinetics of urea cycle enzymes in porcine enterocytes
|
|
6784
|
Mechanism of action of guinea pig liver transglutaminase
|
|
6785
|
Mechanism of action of guinea pig liver transglutaminase
|
|
6786
|
Discrimination of glucose anomers by glucokinase from liver and transplantable insulinoma
|
|
6787
|
Discrimination of glucose anomers by glucokinase from liver and transplantable insulinoma
|
|
6788
|
Discrimination of glucose anomers by glucokinase from liver and transplantable insulinoma
|
|
6789
|
Discrimination of glucose anomers by glucokinase from liver and transplantable insulinoma
|
|
6790
|
The arsonomethyl analogue of adenosine 5'-phosphate. An uncoupler of adenylate kinase
|
|
6791
|
The arsonomethyl analogue of adenosine 5'-phosphate. An uncoupler of adenylate kinase
|
|
6792
|
Random-order ternary complex reaction mechanism of serine acetyltransferase from Escherichia coli
|
|
6793
|
Random-order ternary complex reaction mechanism of serine acetyltransferase from Escherichia coli
|
|
6794
|
Random-order ternary complex reaction mechanism of serine acetyltransferase from Escherichia coli
|
|
6795
|
Random-order ternary complex reaction mechanism of serine acetyltransferase from Escherichia coli
|
|
6796
|
The role of mitochondrially bound arginase in the regulation of urea synthesis: studies with [U-15N4]arginine, ...
|
|
6797
|
The role of mitochondrially bound arginase in the regulation of urea synthesis: studies with [U-15N4]arginine, ...
|
|
6798
|
Site-directed mutagenesis of yeast phosphoglycerate kinase. The 'basic-patch' residue arginine 168
|
|
6799
|
Site-directed mutagenesis of yeast phosphoglycerate kinase. The 'basic-patch' residue arginine 168
|
|
6800
|
Site-directed mutagenesis of yeast phosphoglycerate kinase. The 'basic-patch' residue arginine 168
|
|
6801
|
Site-directed mutagenesis of yeast phosphoglycerate kinase. The 'basic-patch' residue arginine 168
|
|
6802
|
Site-directed mutagenesis of yeast phosphoglycerate kinase. The 'basic-patch' residue arginine 168
|
|
6803
|
Site-directed mutagenesis of yeast phosphoglycerate kinase. The 'basic-patch' residue arginine 168
|
|
6804
|
The arsonomethyl analogue of 3-phosphoglycerate
|
|
6805
|
The arsonomethyl analogue of 3-phosphoglycerate
|
|
6806
|
Purification, characteriziation, and sequence analysis of two alpha-amylase isoforms from azuki bean, Vignia ...
|
|
6807
|
Purification, characteriziation, and sequence analysis of two alpha-amylase isoforms from azuki bean, Vignia ...
|
|
6808
|
Purification, characteriziation, and sequence analysis of two alpha-amylase isoforms from azuki bean, Vignia ...
|
|
6809
|
Purification, characteriziation, and sequence analysis of two alpha-amylase isoforms from azuki bean, Vignia ...
|
|
6810
|
Probing biosynthesis of plant polyketides with synthetic N-acetylcysteamine thioesters
|
|
6811
|
Probing biosynthesis of plant polyketides with synthetic N-acetylcysteamine thioesters
|
|
6812
|
Probing biosynthesis of plant polyketides with synthetic N-acetylcysteamine thioesters
|
|
6813
|
Comparative structural analysis and kinetic properties of lactate dehydrogenases from the four species of ...
|
|
6814
|
Comparative structural analysis and kinetic properties of lactate dehydrogenases from the four species of ...
|
|
6815
|
Comparative structural analysis and kinetic properties of lactate dehydrogenases from the four species of ...
|
|
6816
|
Comparative structural analysis and kinetic properties of lactate dehydrogenases from the four species of ...
|
|
6817
|
Comparative structural analysis and kinetic properties of lactate dehydrogenases from the four species of ...
|
|
6818
|
Comparative structural analysis and kinetic properties of lactate dehydrogenases from the four species of ...
|
|
6819
|
Comparative structural analysis and kinetic properties of lactate dehydrogenases from the four species of ...
|
|
6820
|
Comparative structural analysis and kinetic properties of lactate dehydrogenases from the four species of ...
|
|
6821
|
Comparative structural analysis and kinetic properties of lactate dehydrogenases from the four species of ...
|
|
6822
|
Comparative structural analysis and kinetic properties of lactate dehydrogenases from the four species of ...
|
|
6823
|
Comparative structural analysis and kinetic properties of lactate dehydrogenases from the four species of ...
|
|
6824
|
Comparative structural analysis and kinetic properties of lactate dehydrogenases from the four species of ...
|
|
6825
|
Comparative structural analysis and kinetic properties of lactate dehydrogenases from the four species of ...
|
|
6826
|
Comparative structural analysis and kinetic properties of lactate dehydrogenases from the four species of ...
|
|
6827
|
Comparative structural analysis and kinetic properties of lactate dehydrogenases from the four species of ...
|
|
6828
|
Comparative structural analysis and kinetic properties of lactate dehydrogenases from the four species of ...
|
|
6829
|
Comparative structural analysis and kinetic properties of lactate dehydrogenases from the four species of ...
|
|
6830
|
Comparative structural analysis and kinetic properties of lactate dehydrogenases from the four species of ...
|
|
6831
|
Comparative structural analysis and kinetic properties of lactate dehydrogenases from the four species of ...
|
|
6832
|
Comparative structural analysis and kinetic properties of lactate dehydrogenases from the four species of ...
|
|
6833
|
Structural basis for cold adaptation. Sequence, biochemical properties, and crystal structure of malate ...
|
|
6834
|
Structural basis for cold adaptation. Sequence, biochemical properties, and crystal structure of malate ...
|
|
6835
|
Structural basis for cold adaptation. Sequence, biochemical properties, and crystal structure of malate ...
|
|
6836
|
Structural basis for cold adaptation. Sequence, biochemical properties, and crystal structure of malate ...
|
|
6837
|
Structural basis for cold adaptation. Sequence, biochemical properties, and crystal structure of malate ...
|
|
6838
|
Structural basis for cold adaptation. Sequence, biochemical properties, and crystal structure of malate ...
|
|
6839
|
Structural basis for cold adaptation. Sequence, biochemical properties, and crystal structure of malate ...
|
|
6840
|
Phosphoglucoisomerase-catalyzed interconversion of hexose-phosphates. Isotopic discrimination between hydrogen ...
|
|
6841
|
Phosphoglucoisomerase-catalyzed interconversion of hexose-phosphates. Isotopic discrimination between hydrogen ...
|
|
6842
|
Phosphoglucoisomerase-catalyzed interconversion of hexose-phosphates. Isotopic discrimination between hydrogen ...
|
|
6843
|
Phosphoglucoisomerase-catalyzed interconversion of hexose-phosphates. Isotopic discrimination between hydrogen ...
|
|
6844
|
Phosphoglucoisomerase-catalyzed interconversion of hexose-phosphates. Isotopic discrimination between hydrogen ...
|
|
6845
|
Phosphoglucoisomerase-catalyzed interconversion of hexose-phosphates. Isotopic discrimination between hydrogen ...
|
|
6846
|
Phosphoglucoisomerase-catalyzed interconversion of hexose-phosphates. Isotopic discrimination between hydrogen ...
|
|
6847
|
Phosphoglucoisomerase-catalyzed interconversion of hexose-phosphates. Isotopic discrimination between hydrogen ...
|
|
6848
|
Phosphoglucoisomerase-catalyzed interconversion of hexose-phosphates. Isotopic discrimination between hydrogen ...
|
|
6849
|
Molecular basis for the isozymes of bovine glucose-6-phosphate isomerase
|
|
6850
|
Molecular basis for the isozymes of bovine glucose-6-phosphate isomerase
|
|
6851
|
Molecular basis for the isozymes of bovine glucose-6-phosphate isomerase
|
|
6852
|
Molecular basis for the isozymes of bovine glucose-6-phosphate isomerase
|
|
6853
|
Molecular basis for the isozymes of bovine glucose-6-phosphate isomerase
|
|
6854
|
Molecular basis for the isozymes of bovine glucose-6-phosphate isomerase
|
|
6855
|
Molecular basis for the isozymes of bovine glucose-6-phosphate isomerase
|
|
6856
|
Molecular basis for the isozymes of bovine glucose-6-phosphate isomerase
|
|
6857
|
Comparison of inactivation and conformational changes of D-glyceraldehyde-3-phosphate dehydrogenase during ...
|
|
6858
|
Comparison of inactivation and conformational changes of D-glyceraldehyde-3-phosphate dehydrogenase during ...
|
|
6859
|
Comparison of inactivation and conformational changes of D-glyceraldehyde-3-phosphate dehydrogenase during ...
|
|
6860
|
Comparison of inactivation and conformational changes of D-glyceraldehyde-3-phosphate dehydrogenase during ...
|
|
6861
|
Comparison of inactivation and conformational changes of D-glyceraldehyde-3-phosphate dehydrogenase during ...
|
|
6862
|
Comparison of inactivation and conformational changes of D-glyceraldehyde-3-phosphate dehydrogenase during ...
|
|
6863
|
Comparison of inactivation and conformational changes of D-glyceraldehyde-3-phosphate dehydrogenase during ...
|
|
6864
|
Comparison of inactivation and conformational changes of D-glyceraldehyde-3-phosphate dehydrogenase during ...
|
|
6865
|
Purification, molecular and kinetic properties of glucosamine-6-phosphate isomerase (deaminase) from ...
|
|
6866
|
Purification, molecular and kinetic properties of glucosamine-6-phosphate isomerase (deaminase) from ...
|
|
6867
|
Purification, molecular and kinetic properties of glucosamine-6-phosphate isomerase (deaminase) from ...
|
|
6868
|
Purification, molecular and kinetic properties of glucosamine-6-phosphate isomerase (deaminase) from ...
|
|
6870
|
Arachidonic acid increases the activity of the assembled NADPH oxidase in cytoplasmic membranes and endosomes
|
|
6871
|
Arachidonic acid increases the activity of the assembled NADPH oxidase in cytoplasmic membranes and endosomes
|
|
6872
|
Arachidonic acid increases the activity of the assembled NADPH oxidase in cytoplasmic membranes and endosomes
|
|
6873
|
Arachidonic acid increases the activity of the assembled NADPH oxidase in cytoplasmic membranes and endosomes
|
|
6874
|
Arachidonic acid increases the activity of the assembled NADPH oxidase in cytoplasmic membranes and endosomes
|
|
6875
|
Arachidonic acid increases the activity of the assembled NADPH oxidase in cytoplasmic membranes and endosomes
|
|
6876
|
Arachidonic acid increases the activity of the assembled NADPH oxidase in cytoplasmic membranes and endosomes
|
|
6877
|
Arachidonic acid increases the activity of the assembled NADPH oxidase in cytoplasmic membranes and endosomes
|
|
6878
|
Arachidonic acid increases the activity of the assembled NADPH oxidase in cytoplasmic membranes and endosomes
|
|
6879
|
Arachidonic acid increases the activity of the assembled NADPH oxidase in cytoplasmic membranes and endosomes
|
|
6880
|
Characterization of argininosuccinate lyase (EC 4.3.2.1) from chlamydomonas reinhardtii
|
|
6881
|
Expression of duck lens delta-crystallin cDNAs in yeast and bacterial hosts
|
|
6882
|
Characterization of the multiple forms of duck lens delta-crystallin with endogenous argininosuccinate lyase ...
|
|
6883
|
Characterization of the multiple forms of duck lens delta-crystallin with endogenous argininosuccinate lyase ...
|
|
6884
|
Characterization of the multiple forms of duck lens delta-crystallin with endogenous argininosuccinate lyase ...
|
|
6885
|
Characterization of the multiple forms of duck lens delta-crystallin with endogenous argininosuccinate lyase ...
|
|
6886
|
Characterization of the multiple forms of duck lens delta-crystallin with endogenous argininosuccinate lyase ...
|
|
6887
|
Characterization of the multiple forms of duck lens delta-crystallin with endogenous argininosuccinate lyase ...
|
|
6888
|
Characterization of the multiple forms of duck lens delta-crystallin with endogenous argininosuccinate lyase ...
|
|
6889
|
Characterization of the multiple forms of duck lens delta-crystallin with endogenous argininosuccinate lyase ...
|
|
6890
|
Characterization of the multiple forms of duck lens delta-crystallin with endogenous argininosuccinate lyase ...
|
|
6891
|
Characterization of the multiple forms of duck lens delta-crystallin with endogenous argininosuccinate lyase ...
|
|
6892
|
Argininosuccinate lyase: purification and characterization from human liver
|
|
6893
|
Argininosuccinate lyase: purification and characterization from human liver
|
|
6894
|
Argininosuccinate lyase: purification and characterization from human liver
|
|
6895
|
Subcellular location of glutamine synthetase and urea cycle enzymes in liver of spiny dogfish (squalus ...
|
|
6896
|
Application of the induced-transport test to the mechanism of pig-kidney phosphoglyceromutase
|
|
6897
|
Phosphoglucoisomerase-catalyzed interconversion of hexose phosphates; diastereotopic specificity, isotopic ...
|
|
6898
|
Gluconate dehydratase from the promiscuous Entner-Doudoroff pathway in Sulfolobus solfataricus
|
|
6899
|
Gluconate dehydratase from the promiscuous Entner-Doudoroff pathway in Sulfolobus solfataricus
|
|
6900
|
Mechanism of 1,3-bisphosphoglycerate transfer from phosphoglycerate kinase to glyceraldehyde-3-phosphate ...
|
|
6901
|
The structural basis of substrate promiscuity in glucose dehydrogenase from the hyperthermophilic archaeon ...
|
|
6902
|
The structural basis of substrate promiscuity in glucose dehydrogenase from the hyperthermophilic archaeon ...
|
|
6903
|
The structural basis of substrate promiscuity in glucose dehydrogenase from the hyperthermophilic archaeon ...
|
|
6904
|
The structural basis of substrate promiscuity in glucose dehydrogenase from the hyperthermophilic archaeon ...
|
|
6905
|
The structural basis of substrate promiscuity in glucose dehydrogenase from the hyperthermophilic archaeon ...
|
|
6906
|
The structural basis of substrate promiscuity in glucose dehydrogenase from the hyperthermophilic archaeon ...
|
|
6907
|
The structural basis of substrate promiscuity in glucose dehydrogenase from the hyperthermophilic archaeon ...
|
|
6908
|
The structural basis of substrate promiscuity in glucose dehydrogenase from the hyperthermophilic archaeon ...
|
|
6909
|
The structural basis of substrate promiscuity in glucose dehydrogenase from the hyperthermophilic archaeon ...
|
|
6910
|
The structural basis of substrate promiscuity in glucose dehydrogenase from the hyperthermophilic archaeon ...
|
|
6911
|
The structural basis of substrate promiscuity in glucose dehydrogenase from the hyperthermophilic archaeon ...
|
|
6912
|
The structural basis of substrate promiscuity in glucose dehydrogenase from the hyperthermophilic archaeon ...
|
|
6913
|
The structural basis of substrate promiscuity in glucose dehydrogenase from the hyperthermophilic archaeon ...
|
|
6914
|
The structural basis of substrate promiscuity in glucose dehydrogenase from the hyperthermophilic archaeon ...
|
|
6915
|
Location and properties of glucose dehydrogenase in sporulating cells and spores of Bacillus subtilis
|
|
6916
|
Location and properties of glucose dehydrogenase in sporulating cells and spores of Bacillus subtilis
|
|
6917
|
Location and properties of glucose dehydrogenase in sporulating cells and spores of Bacillus subtilis
|
|
6918
|
Location and properties of glucose dehydrogenase in sporulating cells and spores of Bacillus subtilis
|
|
6919
|
Location and properties of glucose dehydrogenase in sporulating cells and spores of Bacillus subtilis
|
|
6920
|
Location and properties of glucose dehydrogenase in sporulating cells and spores of Bacillus subtilis
|
|
6921
|
Two zebrafish alcohol dehydrogenases share common ancestry with mammalian class I, II, IV, and V alcohol ...
|
|
6922
|
Two zebrafish alcohol dehydrogenases share common ancestry with mammalian class I, II, IV, and V alcohol ...
|
|
6923
|
Two zebrafish alcohol dehydrogenases share common ancestry with mammalian class I, II, IV, and V alcohol ...
|
|
6924
|
Two zebrafish alcohol dehydrogenases share common ancestry with mammalian class I, II, IV, and V alcohol ...
|
|
6925
|
Two zebrafish alcohol dehydrogenases share common ancestry with mammalian class I, II, IV, and V alcohol ...
|
|
6926
|
Feedback regulation of murine pantothenate kinase 3 by coenzyme A and coenzyme A thioesters
|
|
6927
|
Feedback regulation of murine pantothenate kinase 3 by coenzyme A and coenzyme A thioesters
|
|
6928
|
Feedback regulation of murine pantothenate kinase 3 by coenzyme A and coenzyme A thioesters
|
|
6929
|
Feedback regulation of murine pantothenate kinase 3 by coenzyme A and coenzyme A thioesters
|
|
6930
|
Feedback regulation of murine pantothenate kinase 3 by coenzyme A and coenzyme A thioesters
|
|
6931
|
Feedback regulation of murine pantothenate kinase 3 by coenzyme A and coenzyme A thioesters
|
|
6932
|
Feedback regulation of murine pantothenate kinase 3 by coenzyme A and coenzyme A thioesters
|
|
6933
|
Feedback regulation of murine pantothenate kinase 3 by coenzyme A and coenzyme A thioesters
|
|
6934
|
Feedback regulation of murine pantothenate kinase 3 by coenzyme A and coenzyme A thioesters
|
|
6935
|
Feedback regulation of murine pantothenate kinase 3 by coenzyme A and coenzyme A thioesters
|
|
6936
|
Feedback regulation of murine pantothenate kinase 3 by coenzyme A and coenzyme A thioesters
|
|
6937
|
Feedback regulation of murine pantothenate kinase 3 by coenzyme A and coenzyme A thioesters
|
|
6938
|
Feedback regulation of murine pantothenate kinase 3 by coenzyme A and coenzyme A thioesters
|
|
6939
|
Feedback regulation of murine pantothenate kinase 3 by coenzyme A and coenzyme A thioesters
|
|
6940
|
A model of the quaternary structure of enolases, based on structural and evolutionary analysis of the ...
|
|
6941
|
Structural comparison of the enzymatically active and inactive forms of delta-crystallin and the role of ...
|
|
6942
|
Structural comparison of the enzymatically active and inactive forms of delta-crystallin and the role of ...
|
|
6943
|
Nitro analogs of substrates for argininosuccinate Synthetase and argininosuccinate lyase
|
|
6944
|
Nitro analogs of substrates for argininosuccinate Synthetase and argininosuccinate lyase
|
|
6945
|
Nitro analogs of substrates for argininosuccinate Synthetase and argininosuccinate lyase
|
|
6946
|
Nitro analogs of substrates for argininosuccinate Synthetase and argininosuccinate lyase
|
|
6947
|
Biochemical characterization of the class B acid phosphatase (AphA) of Escherichia coli MG1655
|
|
6948
|
Biochemical characterization of the class B acid phosphatase (AphA) of Escherichia coli MG1655
|
|
6949
|
Biochemical characterization of the class B acid phosphatase (AphA) of Escherichia coli MG1655
|
|
6950
|
Biochemical characterization of the class B acid phosphatase (AphA) of Escherichia coli MG1655
|
|
6951
|
Biochemical characterization of the class B acid phosphatase (AphA) of Escherichia coli MG1655
|
|
6952
|
Biochemical characterization of the class B acid phosphatase (AphA) of Escherichia coli MG1655
|
|
6953
|
Biochemical characterization of the class B acid phosphatase (AphA) of Escherichia coli MG1655
|
|
6954
|
Biochemical characterization of the class B acid phosphatase (AphA) of Escherichia coli MG1655
|
|
6955
|
Biochemical characterization of the class B acid phosphatase (AphA) of Escherichia coli MG1655
|
|
6956
|
Biochemical characterization of the class B acid phosphatase (AphA) of Escherichia coli MG1655
|
|
6957
|
Biochemical characterization of the class B acid phosphatase (AphA) of Escherichia coli MG1655
|
|
6958
|
Biochemical characterization of the class B acid phosphatase (AphA) of Escherichia coli MG1655
|
|
6959
|
Biochemical characterization of the class B acid phosphatase (AphA) of Escherichia coli MG1655
|
|
6960
|
Biochemical characterization of the class B acid phosphatase (AphA) of Escherichia coli MG1655
|
|
6961
|
Biochemical characterization of the class B acid phosphatase (AphA) of Escherichia coli MG1655
|
|
6962
|
Biochemical characterization of the class B acid phosphatase (AphA) of Escherichia coli MG1655
|
|
6963
|
Biochemical characterization of the class B acid phosphatase (AphA) of Escherichia coli MG1655
|
|
6964
|
Biochemical characterization of the class B acid phosphatase (AphA) of Escherichia coli MG1655
|
|
6965
|
Biochemical characterization of the class B acid phosphatase (AphA) of Escherichia coli MG1655
|
|
6966
|
Biochemical characterization of the class B acid phosphatase (AphA) of Escherichia coli MG1655
|
|
6967
|
Biochemical characterization of the class B acid phosphatase (AphA) of Escherichia coli MG1655
|
|
6968
|
Biochemical characterization of the class B acid phosphatase (AphA) of Escherichia coli MG1655
|
|
6969
|
Biochemical characterization of the class B acid phosphatase (AphA) of Escherichia coli MG1655
|
|
6970
|
Biochemical characterization of the class B acid phosphatase (AphA) of Escherichia coli MG1655
|
|
6971
|
Determination of Argininosuccinate Lyase and Arginase Activities with an Amino Acid Analyzer
|
|
6972
|
Determination of Argininosuccinate Lyase and Arginase Activities with an Amino Acid Analyzer
|
|
6973
|
Determination of Argininosuccinate Lyase and Arginase Activities with an Amino Acid Analyzer
|
|
6974
|
Determination of Argininosuccinate Lyase and Arginase Activities with an Amino Acid Analyzer
|
|
6975
|
Determination of Argininosuccinate Lyase and Arginase Activities with an Amino Acid Analyzer
|
|
6976
|
Determination of Argininosuccinate Lyase and Arginase Activities with an Amino Acid Analyzer
|
|
6977
|
Betaxanthins as Substrates for Tyrosinase. An Approach to the Role of Tyrosinase in the Biosynthetic Pathway ...
|
|
6978
|
Biochemical characterization and kinetic analysis of duck delta-crystallin with endogenous argininosuccinate ...
|
|
6979
|
Biochemical characterization and kinetic analysis of duck delta-crystallin with endogenous argininosuccinate ...
|
|
6980
|
Biochemical characterization and kinetic analysis of duck delta-crystallin with endogenous argininosuccinate ...
|
|
6981
|
Biochemical characterization and kinetic analysis of duck delta-crystallin with endogenous argininosuccinate ...
|
|
6982
|
Biochemical characterization and kinetic analysis of duck delta-crystallin with endogenous argininosuccinate ...
|
|
6983
|
Biochemical characterization and kinetic analysis of duck delta-crystallin with endogenous argininosuccinate ...
|
|
6984
|
Biochemical characterization and kinetic analysis of duck delta-crystallin with endogenous argininosuccinate ...
|
|
6985
|
Biochemical characterization and kinetic analysis of duck delta-crystallin with endogenous argininosuccinate ...
|
|
6986
|
Biochemical characterization and kinetic analysis of duck delta-crystallin with endogenous argininosuccinate ...
|
|
6987
|
New kinetic parameters for rat liver arginase measured at near-physiological steady-state concentrations of ...
|
|
6988
|
New kinetic parameters for rat liver arginase measured at near-physiological steady-state concentrations of ...
|
|
6989
|
New kinetic parameters for rat liver arginase measured at near-physiological steady-state concentrations of ...
|
|
6990
|
New kinetic parameters for rat liver arginase measured at near-physiological steady-state concentrations of ...
|
|
6991
|
New kinetic parameters for rat liver arginase measured at near-physiological steady-state concentrations of ...
|
|
6992
|
New kinetic parameters for rat liver arginase measured at near-physiological steady-state concentrations of ...
|
|
6993
|
Role of the C-terminal region in the allosteric properties of Escherichia coli phosphofructokinase-1
|
|
6994
|
Role of the C-terminal region in the allosteric properties of Escherichia coli phosphofructokinase-1
|
|
6995
|
Role of the C-terminal region in the allosteric properties of Escherichia coli phosphofructokinase-1
|
|
6996
|
Role of the C-terminal region in the allosteric properties of Escherichia coli phosphofructokinase-1
|
|
6997
|
Detection of kinetically abnormal argininosuccinate synthase in neonatal citrullinemia by conversion of ...
|
|
6998
|
Detection of kinetically abnormal argininosuccinate synthase in neonatal citrullinemia by conversion of ...
|
|
6999
|
Detection of kinetically abnormal argininosuccinate synthase in neonatal citrullinemia by conversion of ...
|
|
7000
|
Detection of kinetically abnormal argininosuccinate synthase in neonatal citrullinemia by conversion of ...
|




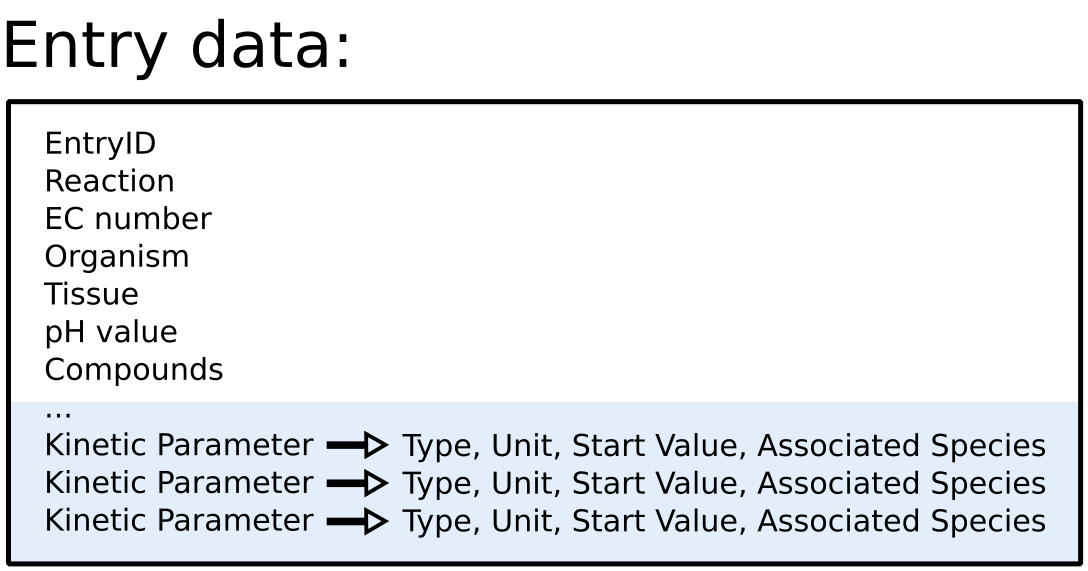
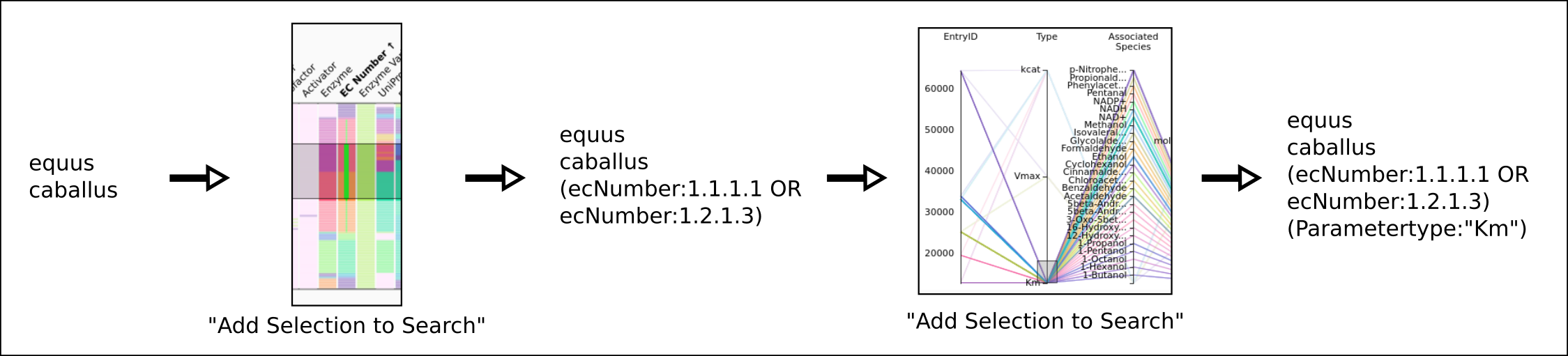 If the current search query is a refinement of the previous query "Use Previous Query (Go Back)" button can be used to perform the previous query again. Only one automatic step back is possible, but the query can be manually manipulated if desired.
If the current search query is a refinement of the previous query "Use Previous Query (Go Back)" button can be used to perform the previous query again. Only one automatic step back is possible, but the query can be manually manipulated if desired.